DielsAlder reactions commonly involve electron-rich dienes and electron-deficient dienophiles: The rate of these reactions generally increases with
Question:
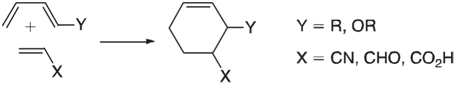
The rate of these reactions generally increases with the π -donor ability of the diene substituent, Y, and with the π -acceptor ability of the dienophile substituent, X. The usual interpretation is that electron donors will push up the energy of the HOMO on the diene and that electron acceptors will push down the energy of the LUMO on the dienophile:
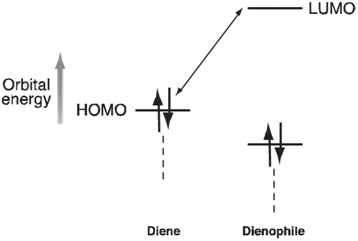
The resulting decrease in the HOMO€“LUMO gap leads to a stronger interaction between diene and dienophile and to a decrease in the activation barrier.
a. Obtain equilibrium geometries for acrylonitrile, 1, 1-dicyanoethylene, cis- and trans-1,2-dicyanoethylene, tricyanoethylene, and tetracyanoethylene using the HF/3-21G model.

Plot the LUMO energy for each dienophile versus the log of the observed relative rate for its addition to cyclopentadiene (listed below the structures in the preceding figure). Is there a reasonable correlation between LUMO energy and relative rate?
b. Obtain transition-state geometries for Diels€“Alder cycloadditions of acrylonitrile and cyclopentadiene and tetracyanoethylene and cyclopentadiene using the HF/3-21G model. Also obtain a geometry for cyclopentadiene. Calculate activation energies for the two reactions. How does the calculated difference in activation energies compare with the experimental difference (based on a value of 7.61 for the difference in the log of the rates and assuming 298 K)?
Step by Step Answer:






