Electron-donating groups on benzene promote electrophilic aromatic substitution and lead preferentially to so-called ortho and para products
Question:
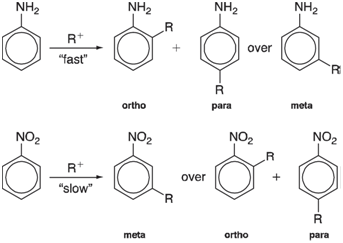
We can expect the first step in the substitution to be addition of the electro-phile, leading to a positively charged adduct:
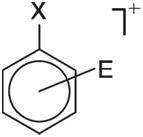
So-called benzenium ions have been characterized spectroscopically and X-ray crystal structures for several are known. Will the stabilities of benzenium ion intermediates anticipate product distribution?
a. Optimize the geometries of benzene, aniline, and nitrobenzene using the HF/3-21G model. You will need their energies to ascertain the relative reactivities of the three substituted benzenes. Also, optimize the geometry of the benzenium ion using the HF/3-21G model. A good guess is a planar six-membered ring comprising five sp2 carbons and an sp3 carbon with bond distances between sp2 carbons intermediate in length between single and double bonds. It should have C2v symmetry. In terms of ring€“bond distances, how does your calculated structure compare with the experimental X-ray geometry of heptamethylbenzenium ion?
b. Optimize the geometries of methyl cation adducts of benzene, aniline (meta and para isomers only), and nitrobenzene (meta and para isomers only) using the HF/3-21G model. Use the calculated structure of parent benzenium ion as a template. Which isomer, meta or para, of the aniline adduct is more stable? Which isomer of the nitrobenzene adduct is more stable? Considering only the lower-energy isomer for each system, order the binding energies of methyl cation addition of benzene, aniline, and nitrobenzene; that is, E(substituted benzene methyl cation adduct) ~E(substituted benzene) ~E(methyl cation). You will need to calculate the energy of the methyl cation using the HF/3-21G model. Which aromatic should be most reactive? Which should be least reactive? Taken as a whole, do your results provide support for the involvement of benzenium ion adducts in electrophilic aromatic substitution? Explain.
Step by Step Answer:






