In this problem, you will solve for the total energy eigenfunctions and eigenvalues for an electron in
Question:
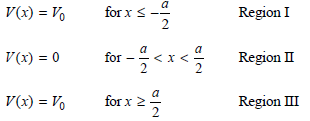
The eigenfunctions must have the following form in these three regions:
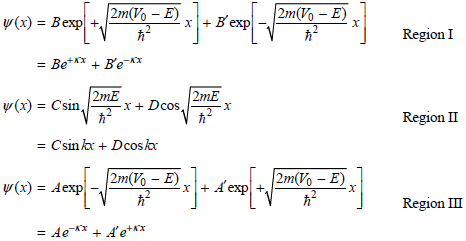
So that the wave functions remain finite at large positive and negative values of x, A€² = B€² = 0. An additional condition must also be satisfied. To arrive at physically meaningful solutions for the eigenfunctions, the wave functions in the separate regions must have the same amplitude and derivatives at the values of x = a/2 and x = ˆ’a/2 bounding the regions. This restricts the possible values for the coefficients A, B, C, and D. Show that applying these conditions gives the following equations:
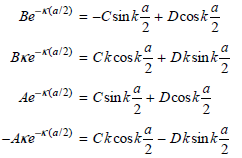
These two pairs of equations differ on the right side only by the sign of one term. We can obtain a set of equations that contain fewer coefficients by adding and subtracting each pair of equations to give
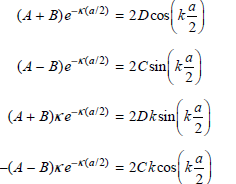
At this point we notice that by dividing the equations in each pair, the coefficients can be eliminated to give
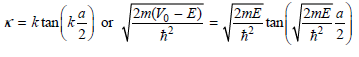
And
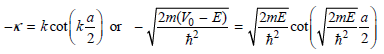
Multiplying these equations on both sides by a/2 gives dimensionless parameters, and the final equations are
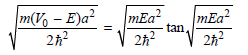
And
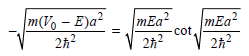
The allowed energy values E must satisfy these equations. They can be obtained by graphing the two sides of each equation against E. The intersections of the two curves are the allowed energy eigenvalues. For the parameters in the caption of Figure 16.1, V0 = 1.20 × 10-18J and a = 1.00 × 10ˆ’9 m, the following two graphs are obtained:
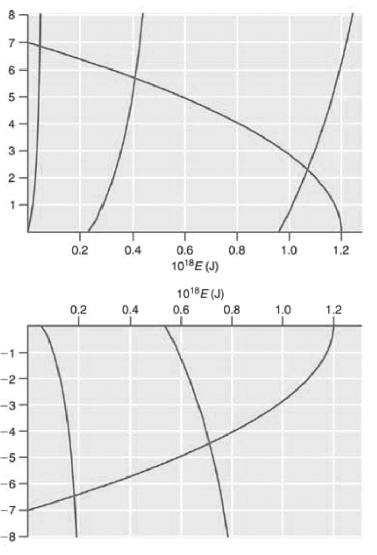
The five allowed energy levels are at 4.61 × 10ˆ’20, 4.09 × 10ˆ’19, and 1.07 × 10ˆ’18 J (top figure), and 1.84 × 10ˆ’19 And 7.13 × 10ˆ’19 J (bottom figure).
a. Given these values, calculate λ for each energy level. Is the relation λ = 2a/n (for n an integer) that arose from the calculations on the infinitely deep box still valid? Compare the values with the corresponding energy level in the infinitely deep box. Explain why the differences arise.
b. Repeat this calculation for V0 = 5.00 × 10 J and a = 0.900 × 10ˆ’9 m. Do you think that there will be fewer or more bound states than for the problem just worked out? How many allowed energy levels are there for this well depth, and what is the energy corresponding to each level?
Step by Step Answer:






