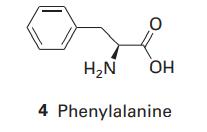
Phenylalanine (Phe, 4) is a naturally occurring amino acid. What is the energy of interaction between its
Question:
Phenylalanine (Phe, 4) is a naturally occurring amino acid. What is the energy of interaction between its phenyl group and the electric dipole moment of a neighbouring peptide group? Take the distance between the groups as 4.0nm and treat the phenyl group as a benzene molecule. The dipole moment of the peptide group is μ =2.7 D and the polarizability volume of benzene is α′=1.04×10−29m3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:





