Singlet carbenes add to alkenes to yield cyclopropanes. Stereochemistry is maintained, meaning that cis- and trans-substituted alkenes
Question:
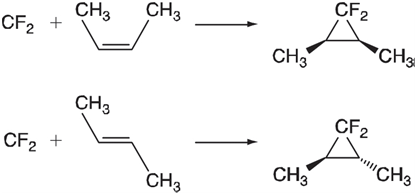
This implies that the two σ bonds are formed more or less simultaneously, without the intervention of an intermediate that would allow cis€“trans isomerization. Locate the transition state for addition of singlet difluorocarbene and ethene using the HF/3-21G model and, following this, calculate vibrational frequencies. When completed, verify that you have in fact found a transition state and that it appears to be on the way to the correct product. What is the orientation of the carbene relative to ethene in your transition state? Is it the same orientation as adopted in the product (1,1-difluorocyclopropane)? If not, what is the reason for the difference? (Consider that the Ï€ electrons on ethene need to go into a low-lying unoccupied molecular orbital on the carbene. Build difluorocarbene and optimize its geometry using the HF/3-21G model, and display the LUMO.)
Step by Step Answer:






