Two empirical equations of state of a real gas are as follows: Evaluate (S/V) T for each
Question:
Two empirical equations of state of a real gas are as follows:
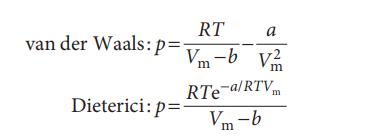
Evaluate (∂S/∂V)T for each gas. For an isothermal expansion, for which kind of gas (also consider a perfect gas) will ΔS be greatest? Explain your conclusion.
Transcribed Image Text:
RT a van der Waals: p=V-b V m m RTe-a/RTVm Dieterici: p= V-b
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
For the van der Waals gas we have SV pVT pTV SV RTVmbVm2bVm aRTVm2bVm SV ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula
Question Posted:
Students also viewed these Sciences questions
-
Consider the isothermal expansion of 1.00 mole of ideal gas at 27oC. The volume increases from 30.0 L to 40.0 L. Calculate q, w, E, H, S, and G for two situations: a. a free expansion b. a reversible...
-
A quantity of an ideal gas undergoes an isothermal expansion at 20 oC and does 3.0 x 103 J of work on its surroundings in the process. (a) Will the entropy of the gas (1) increase, (2) remain the...
-
Explain how the perfect gas equation of state arises by combination of Boyle's law, Charles's law, and Avogadro's principle.
-
What do you think people would say about Corrie from the few quotes we have from her book? What was her personality like? Do you think she handled her incarceration differently than Elie Wiesel?...
-
Summa Manufacturing Company issued $ 900,000 par value, 5%, five- year bonds dated January 1, 2016. The bonds pay interest semiannually each June 30 and December 31. Summa issued the bonds on April...
-
The City of Meringen operates a central garage through an internal service fund to provide garage space and repairs for all city-owned and -operated vehicles. The central garage fund was established...
-
What is the process for starting an appeal?
-
Which portfolio is better diversified, one that contains stock in a dental supply company and a candy company or one that contains stock in a dental supply company and a dairy product company?
-
Please explain how to do the steps PROJECT STEPS 1. 2. 3. 4. 5. 6. 7. As an employee of Catalyst Consultants, you are writing on a report on improving healthcare in the United States. Demote the...
-
Use nodal analysis to find Vo in the circuit of Fig. 3.72. Figure 3.72 For Prob. 3.23. 1 4 30 16
-
Which of F 2 (g) and I 2 (g) is likely to have the higher standard molar entropy at 298K?
-
Discuss the relationships between the various formulations of the Second Law of thermodynamics.
-
What products would you obtain from hydrolysis of a plasmalogens (Problem 27.16) with aqueous NaOH with H3O+?
-
Felix & Company reports the following information. Period 1 Units Produced Total Costs 0 $ 2,020 2 200 2,520 400 3,029 600 3,520 5 800 4,020 6 1,000 4,520 1,200 5,020 1,400 5,520 9 1,600 6,020 10...
-
Explain how the pizza hut influenced sri Lankan customers using the model of buying behavior in sri Lankan market?
-
Consideration is being given to the best option for vaccination against Hepatitis B for diabetic patients. The current standard intervention is three-dose vaccination with Engerix-B. An alternative...
-
10. What is the midpoint between the two points P(x, y) = (-5, 5) and Q(x, y) = (5,-5)?
-
Show how you can generate 6 timing signals in three ways 1) Flip-flops only 2) Flip-flops and AND gates 3) Counter and decoder
-
Let K > 0 be a positive definite n x n matrix. Prove that an n n matrix S satisfies ST K S = I if and only if the columns of S form an orthonormal basis of Rn with respect to the inner product (v,...
-
A handrail, which weighs 120 N and is 1.8 m long. was mounted to a wall adjacent to a small set of steps (Figure P4.26). The support at A has broken, and the rail has fallen about the loose bolt at 8...
-
Discuss the steps involved in the construction of sp 3 , sp 2 , and sp hybrid orbitals.
-
Give the ground-state electron configurations and bond orders of (a) Li 2 , (b) Be 2 , and (c) C 2 .
-
The overlap integral between two H1 s orbitals on nuclei separated by a distance R is S = {1 + (R/a 0 ) + 1/3 (R/a 0 ) 2 }e R/a0 . Plot this function for 0 R < .
-
The manufacturing corporation ABC begun operations on October 1, 2020. Assume the following information concerning plant and equipment, for fiscal years 2020 and 2021. For each numbered item on the...
-
In its income statement for the year ended December 31, 2025, Sandhill Inc reported the following condensed data. Operating expenses $ 719.000 Interest revenue $ 32,000 Cost of goods sold 1,265.000...
-
Wheeling Manufacturing orders 8,000 units of graphite shafts for its production of golf clubs per week. The carrying costs of these shafts are $5 per unit per year and the fixed ordering cost is...

Study smarter with the SolutionInn App


