Use the average bond energies in Table 4.3 to estimate ÎU for the reaction C 2 H
Question:
Table 4.3
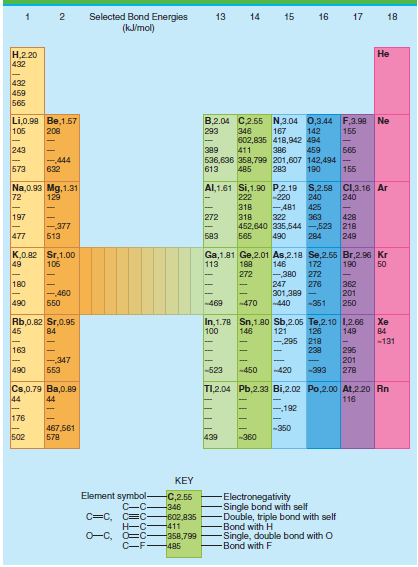
Transcribed Image Text:
Selected Bond Energies (klmol) 13 14 15 16 17 18 Н.220 432 Не 432 459 565 B,2.04 C2.55 N,3.04 0,3.44 F,3.98 Ne Li,0.90 Be,1.57 105 208 293 346 167 142 155 602,835 418,942 494 243 Б65 389 411 386 459 -444 536,636 358,799 201,607 142,494 155 573 632 613 485 283 190 Al,1.61 Si,1.90 P.2.19 S,2.58 C,3.16 Ar Na,0.93 Mg, 1.31 72 129 222 -220 240 240 -481 322 363 452,640 335,544 -523 218 318 425 197 272 318 428 -377 477 583 249 513 565 490 284 K,0.82 Sr,1.00 49 Ga,1.81 Ge,2.01 As,2.18 Se,2.55 Br,2.96 Kr 146 106 113 188 172 190 50 272 -380 272 180 247 362 201 250 276 301,389 - -440 -460 490 Б50 -469 470 -351 In,1.78 Sn,1.80 Sb,2.05 Te,2.10 1,2.66 121 -295 Rb,0.82 Sr,0.95 45 Xe 100 149 84 146 126 84 218 238 -131 163 296 201 278 -,347 553 490 -523 -420 450 -393 TI,2.04 Pb,2.33 Bi,2.02 Po,2.00o At,220 Rn Cs,0.79 Ba,0.89 44 116 44 192 176 -350 467,561 578 502 439 -360 KEY Element symbol- C-C C=C, C=C Electronegativity Single bond with self Double, triple bond with self Bond with H Single, double bond with O -Bond with F C,2.55 -346 602,835 -411 O-C, O=C- -358,799 C-F485
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
U R C C bond energy 6C H bond energy H H bond energy C C bond energy 4C H bond energy U R 346 kJ mo...View the full answer

Answered By

Nimlord Kingori
2023 is my 7th year in academic writing, I have grown to be that tutor who will help raise your grade and better your GPA. At a fraction of the cost on other sites, I will work on your assignment by taking it as mine. I give it all the attention it deserves and ensures you get the grade that I promise. I am well versed in business-related subjects, information technology, Nursing, history, poetry, and statistics. Some software's that I have access to are SPSS and NVIVO. I kindly encourage you to try me; I may be all that you have been seeking, thank you.
4.90+
360+ Reviews
1070+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Basing your answers on the bond dissociation energies in Table 4.3, calculate which of the following reactions are endothermic and which are exothermic: (a) (CH3)2CHOH + HF - (CH3)2CHF + H2O (b)...
-
Use the following data (in kJ/ mol) to estimate ÎH for the reaction S-(g) + e- S2-(g). Include an estimate of uncertainty. S(s) S(g) ÎH = 277 kJ/ mol S(g) + e- S-(g) ÎH = 200. kJ/...
-
The rates of many atmospheric reactions are accelerated by the absorption of light by one of the reactants. For example, consider the reaction between methane and chlorine to produce methyl chloride...
-
A jet is traveling westward with the sun directly overhead (the jet is on a line between the sun and the center of the Earth). How fast must the jet fly in order to keep the sun directly overhead?...
-
Which of the following statements is NOT CORRECT? (a) When a corporation's shares are owned by a few individuals who own most of the stock or are part of the firm's management, we say that the firm...
-
List three major purposes the tax system is meant to serve: a. ___________________________________________________________ b. ___________________________________________________________ c....
-
The red blood cell counts (in grams per deciliter) for a population of adult females can be approximated by a normal distribution, with a mean of 13.5 grams per deciliter and a standard deviation of...
-
Indicate which of the following costs should be expensed when incurred. (a) $13,000 paid to rearrange and reinstall machinery. (b) $200,000 paid for addition to building. (c) $200 Paid for tune-up...
-
A block of 185F iron falls into an insulated container which contains 0.8ft 3 of 70F liquid water. At the same time the water is stirred by a blade attached to a 200W motor. After 10 minutes the...
-
Which of the graphs in Fig. Q25.12 best illustrates the current I in a real resistor as a function of the potential difference V across it? Explain. Figure Q25.12 (a) (b) (c) (d)
-
Using the protein DSC data in Problem P4.10, calculate the enthalpy change between the T = 288 K and T = 318 K. Give your answer in units of kJ per mole. Assume the molecular weight of the protein is...
-
In the compounds below, classify each bond as covalent, polar covalent, or ionic: a) NaBr b) NaOH c) NaOCH 3 d) CH 3 OH e) CH 2 O
-
Which statement is the most accurate? a) Nearly every major economic innovation originated abroad and was then applied in the United States. b) The United States provides a poor environment for...
-
Salgacoar Shippings CEO expressed reservations about the equity financing recommendation that the firms CFO was finalizing for presentation to the board (see problems 6 and 7). He generally agreed...
-
The CFA franc and the birth of the euro. In 1999, the launch of the euro buried the French franc to which the CFA franc was pegged: the CFA franc would now be convertible into euros () rather than...
-
Hong Kong currency board and the Chinese yuan. For the past 25 years Hong Kong has relied on a currency board to peg its currency against the U.S. dollar at HK$7.80 = US$1. Since 2005, the Chinese...
-
If the dollar price of one Russian ruble (RUB) is US$0.03282 = RUB 1 in New York City and at the same time the Russian ruble price of one dollar is 30. 469 in Moscow, show how arbitrageurs could take...
-
ABC Ltd. produces a single product that sells for 75 per unit. Cost data are: (a) Variable manufacturing costs, 35 per unit. (b) Variable selling and administrative expenses, 5 per unit. (c) Fixed...
-
In the case of the gasoline tax, what would happen if the rebate to the consumers were based on their original consumption of gasoline,x,rather than on their final consumption of gasoline, x'?
-
What is the difference between adsorption and absorption?
-
Derive an expression for the rate of disappearance of a species A in a photochemical reaction for which the mechanism is: Hence, show that rate measurements will give only a combination of k2 and k3...
-
Photolysis of Cr (CO)6 in the presence of certain molecules M, can give rise to the following reaction sequence: Suppose that the absorbed light intensity is so weak that! «k4 [Cr (CO) 5M]....
-
Many enzyme-catalyzed reactions are consistent with a modified version of the Michaelis-Menten mechanism in which the second step is also reversible. (a) For this mechanism show that the rate of...
-
The partners mehak and simran share in the profits and losses equally and their capital accounts have credit balance s of 1 5 0 0 0 and 2 5 0 0 0 respectively. The account balances for Mehak and...
-
LNS Corporation reports book profit of $ 2 , 0 0 0 , 0 0 0 . The $ 2 , 0 0 0 , 0 0 0 included $ 1 5 , 0 0 0 of tax exempt interest income, a capital loss of $ 2 , 0 0 0 , depreciation expense of $ 6...
-
Maggie Vitteta, single, works 3 8 hours per week at $ 1 9 . 0 0 an hour. How much is taken out for federal income tax?

Study smarter with the SolutionInn App


