A molecular bond can be modeled as a spring between two atoms that vibrate with simple harmonic
Question:
Transcribed Image Text:
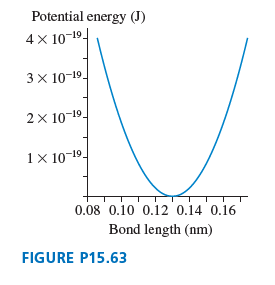
Potential energy (J) 4 x 10-19- 3x 10-19- 2x 10-19- 1 × 10-19- 0.08 0.10 0.12 0.14 0.16 Bond length (nm) FIGURE P15.63
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Solve The potential energy curve of a simple harmonic oscillator is des...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Related Video
simple harmonic motion, in physics, repetitive movement back and forth through an equilibrium, or central, position, so that the maximum displacement on one side of this position is equal to the maximum displacement on the other side. The time interval of each complete vibration is the same
Students also viewed these Physics questions
-
The bumper on a car is made of flexible plastic so that it will compress on impact and cushion the cars occupants during a collision. Suppose a car of mass m = 1200 kg is traveling slowly through a...
-
A flat roof can be modeled as a flat plate insulated on the bottom and placed in the sunlight. If the radiant heat that the roof receives from the sun is 600 W/m2, the convection heat transfer...
-
A coffee maker can be modeled as a heating element (resistance R) connected to the outlet voltage of 120 V (assumed to be dc). The heating element boils small amounts of water at a time as it brews...
-
Refer to the bolt strength problem 17.47. Assume = 6,050 and 5 100. Use the following 24 individual bolt strength observations to answer the questions posed. (a) Prepare a histogram and/or normal...
-
The trial balance of Brentwood Construction at July 31, 2014, appears below Additional data at July 31, 2014: a. Amortization for the period to be recorded: equipment, $2,040; building, $4,210. b....
-
An output interface in a switch is designed using the leaky bucket algorithm to send 8000 bytes/s (tick). If the following frames are received in sequence, show the frames that are sent during each...
-
A chemical plant constructed in 2007 began operating in 2008. In 2010 , the plant is projected to operate at \(90 \%\) of capacity, with (a) Calculate the return on investment (ROI) in 2010 given...
-
A Coast Guard cutter detects an unidentified ship at a distance of 20.0 km in the direction 15.0 east of north. The ship is traveling at 26.0 km/h on a course at 40.0 east of north. The Coast Guard...
-
4) Determine the support reactions for the system shown. There is a pin at A and a roller at B. The external force of 1000 N is applied at x = 2 meters. A F = 1000 N 4 meters B
-
Games Galore has provided its condensed financial statements for the year ended December 31, 2016. The Controller has asked you to calculate liquidity, solvency, and profitability ratios that...
-
FIGURE P15.62 is a top view of an object of mass m connected between two stretched rubber bands of length L. The object rests on a frictionless surface. At equilibrium, the tension in each rubber...
-
A penny rides on top of a piston as it undergoes vertical simple harmonic motion with an amplitude of 4.0 cm. If the frequency is low, the penny rides up and down without difficulty. If the frequency...
-
Show that : C M 2 (R) given by for a, b R gives an isomorphism of C with the subring [C] of M 2 (R). p(a + bi) = D -b a
-
What are the fundamental functions of the nucleus in a eukaryotic cell?
-
Consider the following BWT: smnpbnnaaaaa$a Generate the LastToFirst (L2F) array from this BWT. What are the values of the L2F array? Order them such that the top item is the first element of the L2F...
-
In the article by Bohan and Smyth - The effect of schedule thinning on student behavior during the caught being good game : What are the limitations of the study? List at least two limitations. NOTE:...
-
As the US government prepares to ban TikTok, and on the expectation that Canada may follow suit, you have developed a similar alternative social media video hosting platform. You see an opportunity...
-
We are all used to wages rising. However, what could possibly cause wages to decrease? What signs should we look for to ensure we are not in that profession?
-
Can you explain why some transport-layer packets may be lost in the Internet?
-
Making use of the tables of atomic masses, find the velocity with which the products of the reaction B10 (n, ) Li7 come apart; the reaction proceeds via interaction of very slow neutrons with...
-
Complete the calculation of the mass of Earth as outlined in Section 2.7.
-
The Kingda Ka roller coaster in New Jersey is the worlds tallest ride of its kind. As the passenger cars are launched from rest at the start, they are accelerated uniformly to a speed of 57 m/s (128...
-
During our discussion of the motion of a falling body near Earths surface, we said that the gravitational force acting on itits weightis constant. But the law of universal gravitation tells us that...
-
A friend owes you $500 in 1 year from now, and then $1000 2 years from now. Both loans are at 5%. If you want to replace both of these loans with ONE PAYMENT NOW, how much would your friend give you ?
-
If the amount of retained earnings at the beginning of the year was $ 3 0 , 0 0 0 , and $ 1 2 , 0 0 0 in dividends is paid during the year, calculate net income for the year.
-
On January 2, 20Y4, Whitworth Company acquired 33% of the outstanding stock of Aloof Company for $330,000. For the year ended December 31, 20Y4, Aloof Company earned income of $86,000 and paid...

Study smarter with the SolutionInn App


