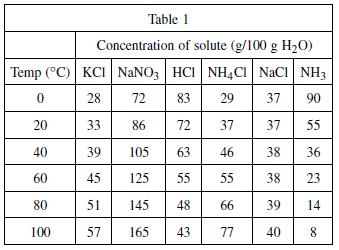
According to Table 1, HCl would most likely have which of the following concentrations at 70
Question:
According to Table 1, HCl would most likely have which of the following concentrations at 70◦C?
A solute is any substance that is dissolved in another substance, which is called the solvent. A student tested the solubility (a measure of how much solute will dissolve into the solvent) of six different substances. The solubility of a substance at a given temperature is defined as the concentration of the dissolved solute that is in equilibrium with the solvent. Table 1 represents the concentration of dissolved substances in 100 grams of water at various temperatures. The concentrations are expressed in grams of solute per 100 grams of water.
 F. 25.5 g/100g H2O
F. 25.5 g/100g H2O
G. 37.0 g/100g H2O
H. 48.5 g/100g H2O
J. 51.5 g/100g H2O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





