In contrast to the situation of Exercise 40, experiments show that the reaction H 2 + Br
Question:
In contrast to the situation of Exercise 40, experiments show that the reaction H2 + Br2 → 2HBr satisfies the rate law
![d[HBr] dt k[H2][Br]/?](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1553/7/7/9/7545c9ccc2af30cd1553762540508.jpg)
and so for this reaction the differential equation becomes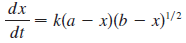
where x = [HBr] and a and b are the initial concentrations of hydrogen and bromine.
(a) Find x as a function of t in the case where a = b. Use the fact that x(0) = 0.
(b) If a > b, find t as a function of x.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





