A mixture containing only aluminum tetrafl uoroborate, Al(BF 4 ) 3 (FM 287.39), and magnesium nitrate, Mg(NO
Question:
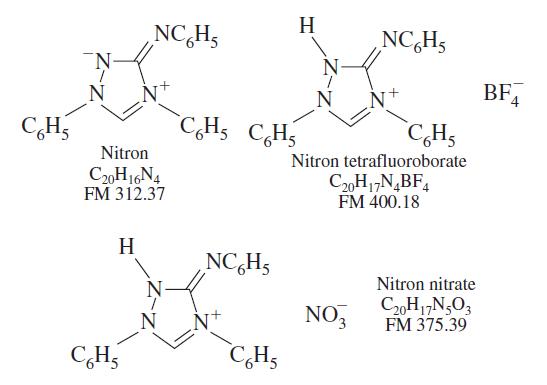
A mixture containing only aluminum tetrafl uoroborate, Al(BF4)3 (FM 287.39), and magnesium nitrate, Mg(NO3)2 (FM 148.31), weighed 0.282 8 g. It was dissolved in 1 wt% HF(aq) and treated with nitron solution to precipitate a mixture of nitron tetrafluoroborate and nitron nitrate weighing 1.322 g. Find the wt% Mg in the original solid mixture.
Transcribed Image Text:
H NC,Hs NC,H5 BF, CH5 C,Hs CH5 CH; Nitron Nitron tetrafluoroborate C20H16N4 FM 312.37 C2,H1,N,BF, FM 400.18 H NC,H5 Nitron nitrate NO, C20H17N,O3 FM 375.39 N CH, C,H,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
Nitronis C 20 H 16 N 4 Nitron tetrafluoroborate is C 20 H 16 N 4 BF 4 FM 39918 Nitro...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A mixture containing only Al2O3 (FM 101.96) and Fe2O3 (FM 159.69) weighs 2.019 g. When heated under a stream of H2, Al2O3 is unchanged, but Fe2O3 is converted into metallic Fe plus H2O(g). If the...
-
A solid mixture weighing 0.5485 g contained only ferrous ammonium sulfate hexahydrate and ferrous chloride hexahydrate. The sample was dissolved in 1M H2SO4, oxidized to Fe3+ with H2O2, and...
-
A mixture weighing 7.290 mg contained only cyclohexane, C6H12(FM 84.159), and oxirane, C2H4O (FM 44.053). When the mixture was analyzed by combustion analysis, 21.999 mg of CO2 (FM 44.010) were...
-
A force F = (3.00 N)i + (7.00 N)j + (7.00 N)k acts on a 2.00 kg mobile object that moves from an initial position of di = (3.00 m)i (2.00 m)i + (5.00 m)k to a final position of df = (5.00 m)i +...
-
Should discussions of employee job performance be separated from salary considerations?
-
Suppose the Titanic Ice Cube Co.s dividend grows at a 20 percent rate for the next three years. Thereafter, it grows at a 12 percent rate. What value would we place on Titanic assuming a 15 percent...
-
The standard coupling between railroad cars must be capable of withstanding the maximum tensile force exerted on any coupling in a given train. (a) If a locomotive is pulling ten cars and speeding...
-
Adria Lopezs two departments, computer consulting services and computer workstation furniture manufacturing, have each been profitable. Adria has heard of the balanced scorecard and wants you to...
-
c) Compute the net quantities needed if there are 25 of the base and 75 of the clamp in stock. Base: Spring: Clamp: Housing: Handle: Casting: Bearing: Shaft: units (enter your response as a whole...
-
Draw a structured flowchart or write structured pseudocode describing how to decide what college to attend. Include at least two decisions and two loops.
-
Why is high relative supersaturation undesirable in a gravimetric precipitation?
-
Explain what is done in thermogravimetric analysis.
-
Use Figure to calculate the critical angles for total internal reflection for light initially in silicate flint glass that is incident on a glassair interface if the light is (a) Violet light of...
-
Could you expound upon the dynamics and functions of both money and capital markets, elucidating their respective roles within the broader financial ecosystem?
-
Jaxon purchased his first home for $155,600. He obtained a 30-year, fixed-rate mortgage with an interest rate of 7.15% in the amount of $150,400. The seller paid $2,180.45 in property taxes for the...
-
A motorcyclist drives along a straight road from A to B in 12 s. 840 m The average speed of the motorcyclist is?
-
Let V be a vector space over a field k, let WCV be any subset, and let kW = {aw + a2W2 + ... + anwn | ai Ek, w; E W}. Show that kW is a subspace of V. Problem 4. Let k be a field and n E N. Show that...
-
A stock has just paid a $0.50 dividend from earnings per share of $1.50. The stock's beta is 0.8, the risk-free rate is 1.25% and the expected return on the market is 8%. If the dividend is expected...
-
Muons are created by cosmic-ray collisions at an elevation h (as measured in Earth's frame of reference) above Earth's surface and travel downward with a constant speed of 0.990c. During a time...
-
Use a calculator to evaluate the expression. Round your result to the nearest thousandth. V (32 + #)
-
State whether the errors in (a) - (d) are random or systematic: (a) A 25-mL transfer pipet consistently delivers 25.031 0.009 mL. (b) A 10 - mL buret consistently delivers 1.98 0.01 mL when drained...
-
Cheryl, Cynthia, Carmen, and Chastity shot the targets below at Girl Scout camp. Match each target with the proper description. (a) Accurate and precise (b) Accurate but not precise (c) Precise but...
-
Rewrite the number 3.123 56 ( 0.167 89%) in the forms (a) number ( absolute uncertainty) and (b) number ( percent relative uncertainty) with an appropriate number of digits.
-
A significant drop in gasoline prices has encouraged more consumers to purchase cars. This has led to an increase in demand for car mechanics' services; however, here are not currently enough...
-
In 1930 the Australian Football League (AFL), at the time the Victorian Football League (VFL), implemented a price ceiling wage on all AFL players known as the Coulter Law. The ceiling was 3 pounds,...
-
Consider this scatter plot of the average inflation-adjusted U.S. domestic airfare price from the fourth quarter of every year from 1995 to 2014. Scatter plot of the average inflation-adjusted U.S....

Study smarter with the SolutionInn App


