a. Write the half-reactions for a H2-O2H 2 -O 2 fuel cell. Find the theoretical cell voltage
Question:
a. Write the half-reactions for a H2-O2H2-O2 fuel cell. Find the theoretical cell voltage at 25°C 25°C if PH2=1.0 bar, PH2=1.0 bar, PO2=0.2 bar PO2=0.2 bar and [H+]=0.5 M[H+] = 0.5 M at both electrodes. (Real fuel cells operate at temperatures of 60° -1 000°C 60° -1 000°C and produce ~0.7 V.~0.7 V.)
b. If the cell is 70% 70% efficient at converting chemical energy into electrical energy, and a stack of cells produces 20 kW 20kW at 220 V, 220 V how many grams of H2H2 are consumed in an hour?
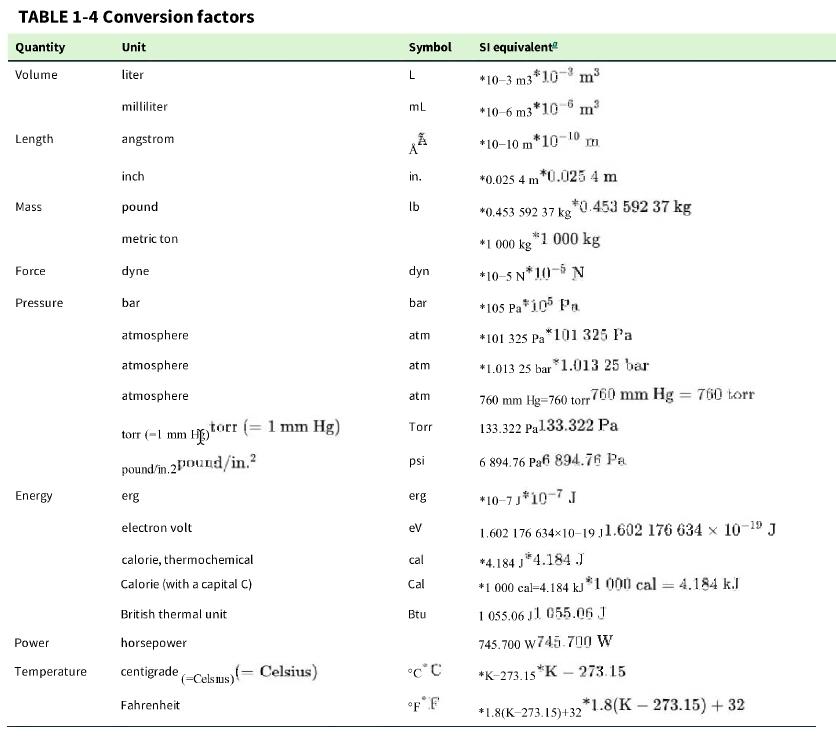
c. In the United States, engines are frequently rated in “horsepower.” Use Table 1-4 to convert 20 kW 20 kW to horsepower.
Table 1-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy
Question Posted:





