Consider a reversible redox reaction at the surface of an electrode. Reversible means that the reaction is
Question:
Consider a reversible redox reaction at the surface of an electrode. “Reversible” means that the reaction is fast enough so that reactants and products maintain equilibrium at the electrode surface consistent with the Nernst equation.
a. Why are there peaks (not plateaus) in cyclic voltammetry at a macroscopic planar electrode in Figure 17-19? Electrode size is millimeters.
b. Why are there plateaus (not peaks) in cyclic voltammetry at a microscopic electrode in Figure 17-35? Electrode size is micrometers.
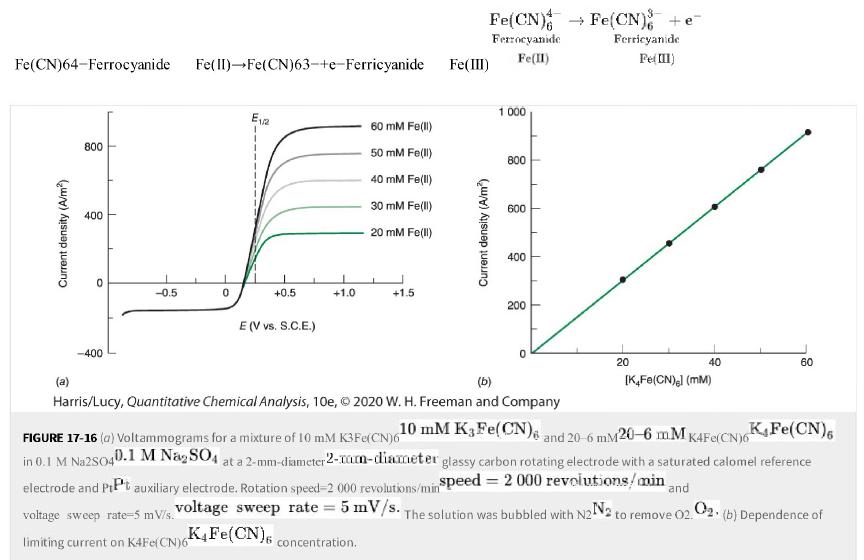
c. Why are there plateaus instead of peaks in cyclic voltammetry at a macroscopic rotating disk electrode in Figure 17-16?
Figure 17-19
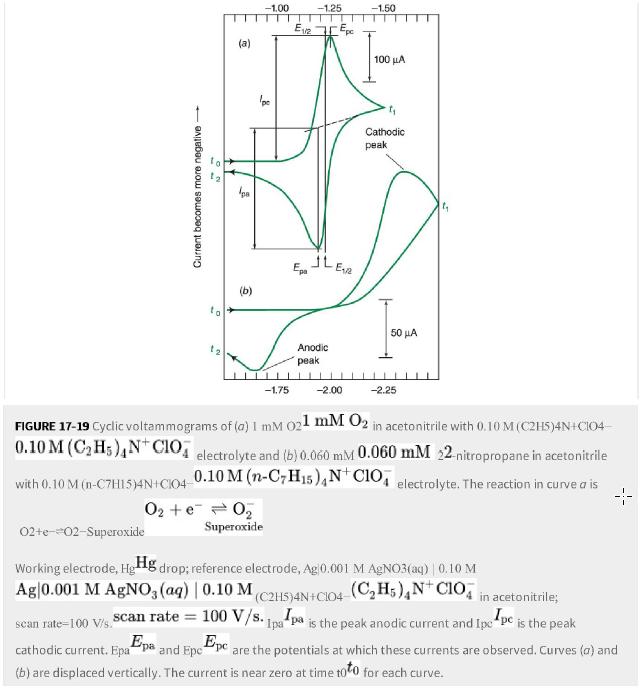
Figure 17-35
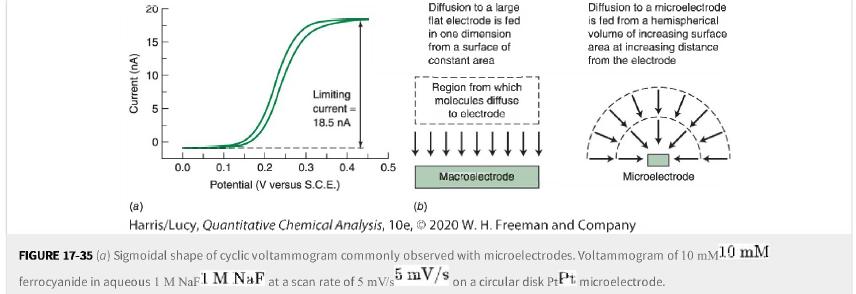
Figure 17-16
Step by Step Answer:

Quantitative Chemical Analysis
ISBN: 9781319164300
10th Edition
Authors: Daniel C. Harris, Charles A. Lucy





