Cu + reacts with neocuproine to form the colored complex (neocuproine)2Cu + , with an absorption maximum
Question:
Cu+ reacts with neocuproine to form the colored complex (neocuproine)2Cu+, with an absorption maximum at 454 nm. Neocuproine is particularly useful because it reacts with few other metals. The copper complex is soluble in 3-methyl-1-butanol (isoamyl alcohol), an organic solvent that does not dissolve appreciably in water. In other words, when isoamyl alcohol is added to water, a two-layered mixture results, with the denser water layer at the bottom. If (neocuproine) 2Cu+ is present, virtually all of it goes into the organic phase. For the purpose of this problem, assume that the isoamyl alcohol does not dissolve in the water at all and that all of the colored complex will be in the organic phase. Suppose that the following procedure is carried out:
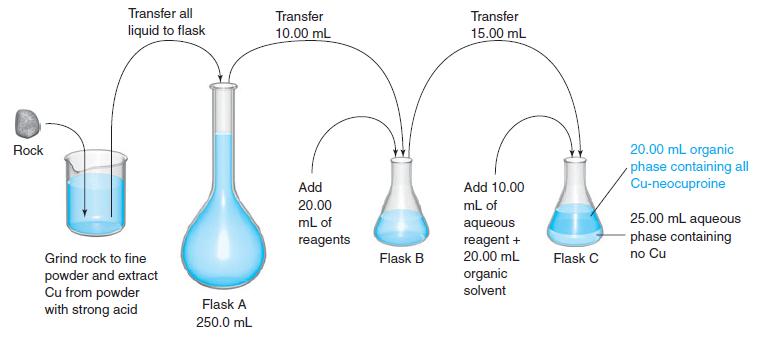
1. A rock containing copper is pulverized, and all metals are extracted from it with strong acid. The acidic solution is neutralized with base and made up to 250.0 mL in flask A.
2. Next, 10.00 mL of the solution are transferred to flask B and treated with 10.00 mL of reducing agent to convert Cu2+ to Cu+. Then 10.00 mL of buffer are added so that the pH is suitable for complex formation with neocuproine.
3. 15.00 mL of this solution are withdrawn and placed in flask C. To the flask are added 10.00 mL of an aqueous solution containing neocuproine and 20.00 mL of isoamyl alcohol. After the mixture has been shaken well and the phases allowed to separate, all (neocuproine)2Cu+ is in the organic phase.
4. A few milliliters of the upper layer are withdrawn, and the absorbance at 454 nm is measured in a 1.00-cm cell. A blank carried through the same procedure gives an absorbance of 0.056.
(a) Suppose that the rock contained 1.00 mg of Cu. What will be the concentration of Cu (mol/L) in the isoamyl alcohol phase?
(b) If the molar absorptivity of (neocuproine)2Cu+ is 7.90 × 103 M-1 cm-1, what will be the observed absorbance? Remember that a blank carried through the same procedure gave an absorbance of 0.056.
(c) A rock is analyzed and found to give a final absorbance of 0.874 (uncorrected for the blank). How many milligrams of Cu are in the rock?
Step by Step Answer:






