For a solution of Ni 2+ and ethylenediamine, the following equilibrium constants apply at 20C: Calculate the
Question:
For a solution of Ni2+ and ethylenediamine, the following equilibrium constants apply at 20°C:
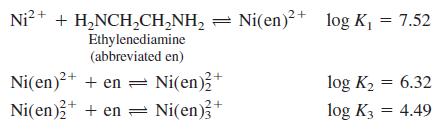
Calculate the concentration of free Ni2+ in a solution prepared by mixing 0.100 mol of en plus 1.00 mL of 0.010 0 M Ni2+ and diluting to 1.00 L with dilute base (which keeps all the en in its unprotonated form. Assume that nearly all nickel is in the form Ni(en)32+, so [Ni(en)32+] = 1.00 × 10-5 M. Calculate the concentrations of Ni(en)2+ and Ni(en)22+ to verify that they are negligible in comparison with Ni(en)32+.

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





