Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. A simplified flowsheet for the Galvanox process is shown below. This process is being developed at UBC. Chaclcopyrite is the most abundant mineral
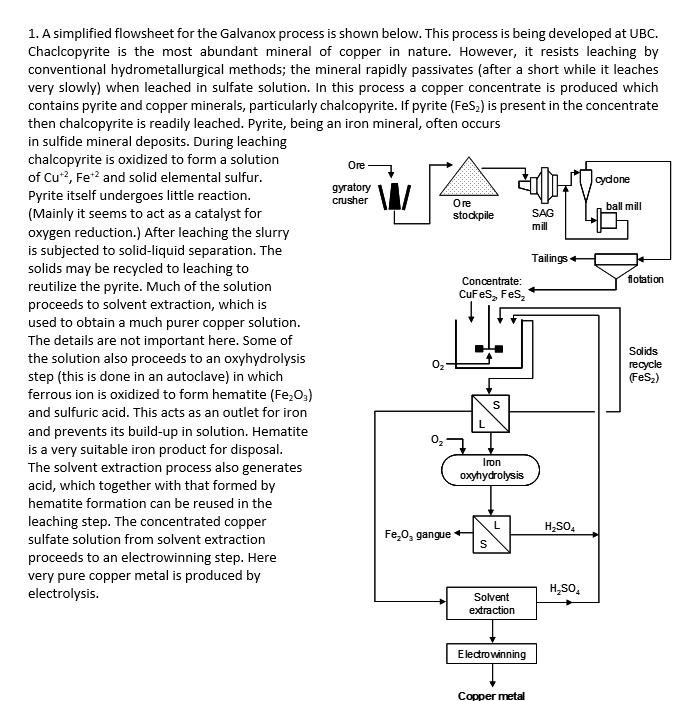

1. A simplified flowsheet for the Galvanox process is shown below. This process is being developed at UBC. Chaclcopyrite is the most abundant mineral of copper in nature. However, it resists leaching by conventional hydrometallurgical methods; the mineral rapidly passivates (after a short while it leaches very slowly) when leached in sulfate solution. In this process a copper concentrate is produced which contains pyrite and copper minerals, particularly chalcopyrite. If pyrite (FeS2) is present in the concentrate then chalcopyrite is readily leached. Pyrite, being an iron mineral, often occurs in sulfide mineral deposits. During leaching chalcopyrite is oxidized to form a solution of Cu+2, Fe+ and solid elemental sulfur. Pyrite itself undergoes little reaction. (Mainly it seems to act as a catalyst for oxygen reduction.) After leaching the slurry is subjected to solid-liquid separation. The solids may be recycled to leaching to reutilize the pyrite. Much of the solution proceeds to solvent extraction, which is used to obtain a much purer copper solution. The details are not important here. Some of the solution also proceeds to an oxyhydrolysis step (this is done in an autoclave) in which ferrous ion is oxidized to form hematite (Fe2O3) and sulfuric acid. This acts as an outlet for iron and prevents its build-up in solution. Hematite is a very suitable iron product for disposal. The solvent extraction process also generates acid, which together with that formed by hematite formation can be reused in the leaching step. The concentrated copper sulfate solution from solvent extraction proceeds to an electrowinning step. Here very pure copper metal is produced by electrolysis. Ore cydone gyratory crusher Ore ball mill stockpile SAG mill Tailings flotation Concentrate: CuFeS2 FeS2 S Iron oxyhydrolysis HSO4 FeO, gangue S HSO4 Solvent extraction Solids recycle (FeS2) Electrowinning Copper metal Answer the following questions related to the flowsheet. (a) Write the balanced chemical reaction in ionic and neutral forms for the leaching of chalocopyrite. (b) Referring to the generalized hydrometallurgical flowsheet on p. 30 of the Introduction to Extractive Metallurgy course notes, identify the parts of the flowsheet that correspond to mineral separation, leaching, solution purification and metal production. Use the flowsheet on the page below, circle and label the appropriate parts of the flowsheet. Hand this in with your completed assignment. (c) Referring to the generalized metallurgical flowsheet that follows, indicate which route corresponds most closely to this process. You may indicate just the number in your assignment paper. (d) Leaching requires 4 moles of H*/mole of CuFeS2. What fraction of the required acid is produced by the oxyhydrolysis step? (Consider the balanced reaction that forms Fe2O3.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


