Question: 12.42 Complete these equations. (a) (b) (c) CH3(CH2)5CH=CH2+HI (d) (e) CH3CH=CHCH2CH3+H2OH2SO4 (f) CH2=CHCH2CH2CH3+H2OH2SO4 12.44 Draw a structural formula for the product formed by treatment of
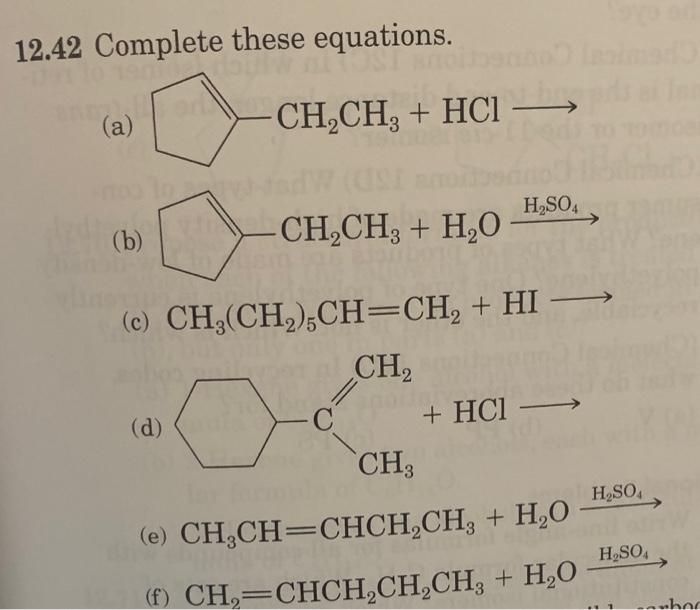



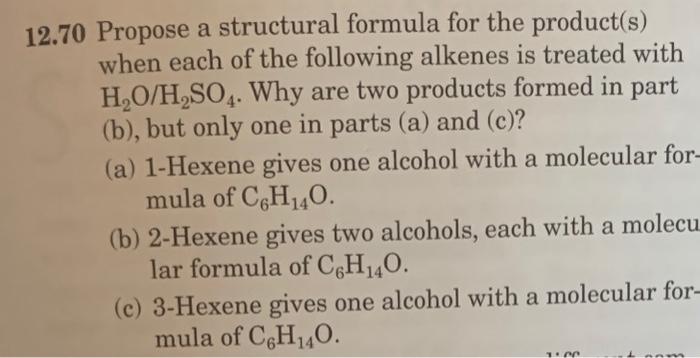
12.42 Complete these equations. (a) (b) (c) CH3(CH2)5CH=CH2+HI (d) (e) CH3CH=CHCH2CH3+H2OH2SO4 (f) CH2=CHCH2CH2CH3+H2OH2SO4 12.44 Draw a structural formula for the product formed by treatment of 2-methyl-2-pentene with each reagent. (a) HCl (b) H2O in the presence of H2SO4 12.56 Show how to convert 1-butene to these compounds. (a) Butane (b) 2-Butanol (c) 2-Bromobutane (d) 1,2-Dibromobutane 12.66 Write line-angle formulas for all compounds with the molecular formula C4H8. Which are sets of constitutional isomers? Which are sets of cis-trans isomers? 12.70 Propose a structural formula for the product(s) when each of the following alkenes is treated with H2O/H2SO4. Why are two products formed in part (b), but only one in parts (a) and (c)? (a) 1-Hexene gives one alcohol with a molecular for mula of C6H14O. (b) 2-Hexene gives two alcohols, each with a molecu lar formula of C6H14O. (c) 3-Hexene gives one alcohol with a molecular formula of C6H14O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


