Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A temperature change from 15C to 35C corresponds to what incremental change in F? (36) A steel wire, 150 m long at 10C, has
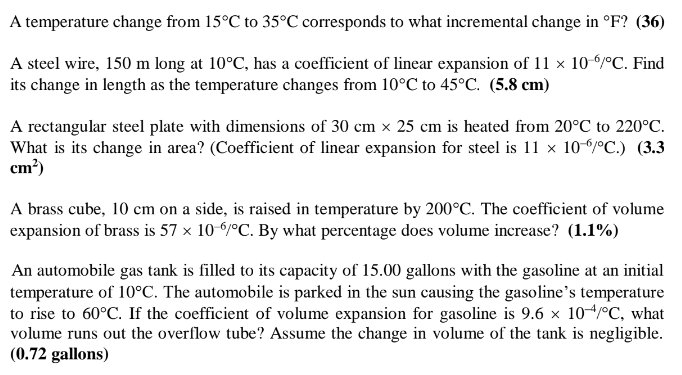
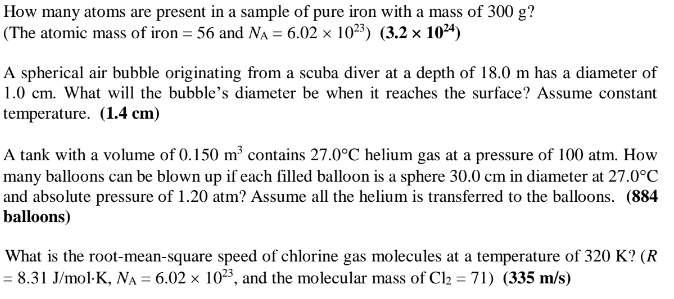

A temperature change from 15C to 35C corresponds to what incremental change in F? (36) A steel wire, 150 m long at 10C, has a coefficient of linear expansion of 11 10-6/C. Find its change in length as the temperature changes from 10C to 45C. (5.8 cm) A rectangular steel plate with dimensions of 30 cm 25 cm is heated from 20C to 220C. What is its change in area? (Coefficient of linear expansion for steel is 11 10/C.) (3.3 cm) A brass cube, 10 cm on a side, is raised in temperature by 200C. The coefficient of volume expansion of brass is 57 10-6/C. By what percentage does volume increase? (1.1%) An automobile gas tank is filled to its capacity of 15.00 gallons with the gasoline at an initial temperature of 10C. The automobile is parked in the sun causing the gasoline's temperature to rise to 60C. If the coefficient of volume expansion for gasoline is 9.6 104/C, what volume runs out the overflow tube? Assume the change in volume of the tank is negligible. (0.72 gallons) How many atoms are present in a sample of pure iron with a mass of 300 g? (The atomic mass of iron = 56 and NA = 6.02 1023) (3.2 1024) A spherical air bubble originating from a scuba diver at a depth of 18.0 m has a diameter of 1.0 cm. What will the bubble's diameter be when it reaches the surface? Assume constant temperature. (1.4 cm) A tank with a volume of 0.150 m contains 27.0C helium gas at a pressure of 100 atm. How many balloons can be blown up if each filled balloon is a sphere 30.0 cm in diameter at 27.0C and absolute pressure of 1.20 atm? Assume all the helium is transferred to the balloons. (884 balloons) What is the root-mean-square speed of chlorine gas molecules at a temperature of 320 K? (R = 8.31 J/molK, NA = 6.02 1023, and the molecular mass of Cl = 71) (335 m/s) A quantity of a monatomic ideal gas expands to twice the volume while maintaining the same pressure. If the internal energy of the gas were Uo before the expansion, what is it after the expansion? (2 Uo)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


