Question
Ammonia is used for fertilizer production and has been critical to successful agriculture. A steady-state chemical pro- cess is used to convert nitrogen (N2)
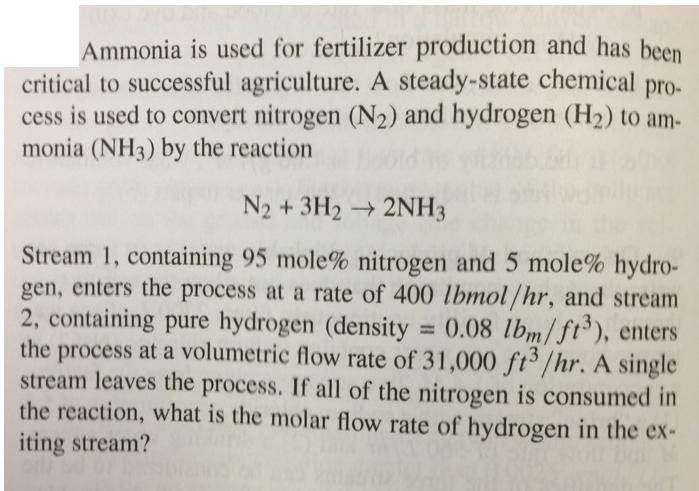
Ammonia is used for fertilizer production and has been critical to successful agriculture. A steady-state chemical pro- cess is used to convert nitrogen (N2) and hydrogen (H) to am- monia (NH3) by the reaction N2 + 3H2 2NH3 Stream 1, containing 95 mole % nitrogen and 5 mole% hydro- gen, enters the process at a rate of 400 lbmol/hr, and stream 2, containing pure hydrogen (density = 0.08 lbm/ft), enters the process at a volumetric flow rate of 31,000 ft3/hr. A single stream leaves the process. If all of the nitrogen is consumed in the reaction, what is the molar flow rate of hydrogen in the ex- iting stream?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



