Question: Answer all questions on page 2-4 Hydrocarbons This class of compounds is referred to as Aliphatic. Alkanes have the formula CnH2n+2 and their name ends
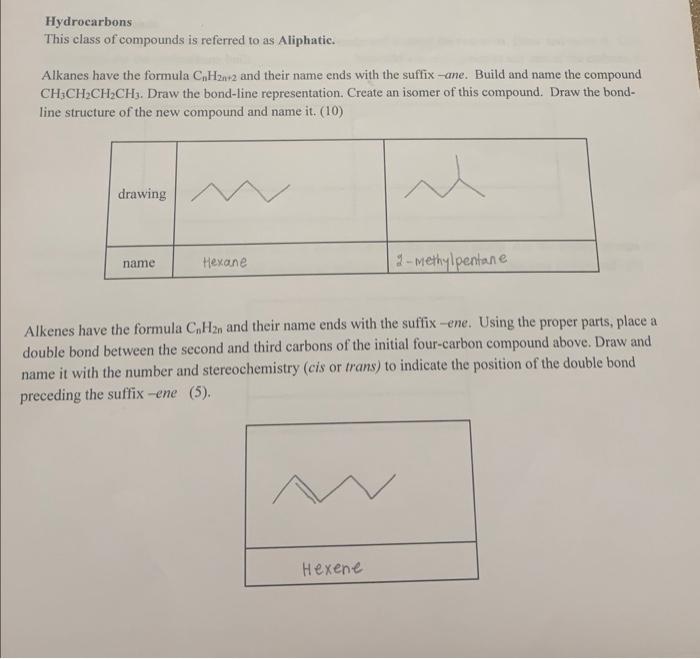



Hydrocarbons This class of compounds is referred to as Aliphatic. Alkanes have the formula CnH2n+2 and their name ends with the suffix-ane. Build and name the compound CH3CH2CH2CH3. Draw the bond-line representation. Create an isomer of this compound. Draw the bondline structure of the new compound and name it. (10) Alkenes have the formula CnH2n and their name ends with the suffix -ene. Using the proper parts, place a double bond between the second and third carbons of the initial four-carbon compound above. Draw and name it with the number and stereochemistry (cis or trans) to indicate the position of the double bond preceding the suffix -ene (5). Akynes hine the formula CaHs at and their narne ends with the swifie whe. Uuine the - Boper connecting: pur, place is triple bond berween the second and thind carbons of your initial allewe. Draw and name thin: coumpound, again with the number precediegr the ahe. What is the cerpinical formule? (7) Cyelie Compounds Remove a hydrogen from each terminal of your molecule and eonnect the two ends. Draw and name it. Give the formula for the cycloalkane built the second bos draw and name this. (12) Add a second methyl group to a carbon next to the earbon with the methyl group. Are the methyl groups cis or trans to each other? Use dashes and wedges to indicate. What is the name of this new compound? (5) Comyounds that coutain heteraatems contnin aioms other than catbon and bydrogen. Haloalkanes are an example, and have the formuln Culhe- 1X, where X is the heleroatom. Butld a five-earbon straipht chain. alkane and add a - Cl to ose of the end carboge. Nimene and draw it. Now move the -Cl to the necond carbon, name and draw it. In each formula box label I' (primary) or Z (secondary) for the number of carbon atoms conneched to the carbon with the Cl. Otve the formala of the compound (14) Amines are nitrogen-containing compounds. They are nansed using N - to indicate the diphatic groups connected to the nitrogen atom and their name ends with the suffix -amine. Build a five-carbon straight chain alkane compound and add the amine group - NH2 to one end. Name and draw it, Move one CH3 to the N, draw and name this, Label each N as I or Z. Give the formuln of the compound (14) Alcohols are oxygen-containing compounds with the generic formula ROH. The R-part is she hydrocarbon part of the molecule. The name of an aleohol ends with the suftix o. Add a hydroxyl group OH to one terminal carbon of a five-carbon straight chain alkane compound. Draw and name it. Now move the OH to the second carbon, draw and name it. Label each OH as I or 2 . Give the formula for the alcohol (14) Bliters are compouands with the ecneral formula ROR, and contain a carbon-oxygen-cuzbon connection Their name includes the woed ether. Mhve the oxygen atoen ftom your primary alcohal from the terminal carbon atomi to butween the firs and second carbon atorne. Draw and nome this new comporand. IUPAC flnternational ution of pure and applied cherniatry) names involve using albory derivatives. For example, ah OCH group is called a methoxy group. Also give the IUPAC name for the ether. (6) Aldehydes are compounds that contain a carbonyl group CO that contains an attached bydrogen atotn. The name of an aldehyde ends with the suffix -al. Convert the terminal carbon of your initial four-carbon alkane to a carbonyl carbon. Draw and name it. Give the formula for the aldehyde (7) Ketones are compounds that contain an internal carbonyl group with the gencral formula RC=OR. The name of a ketone ends with the suffix -one. Move the C=O from the end of your aldehyde to the position of a second carbon. Name and draw it. Give the formula for the ketone (6)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


