Question: For the catalytic decomposition of some alcohols into alkenes and water, the fol- lowing results have been obtained: Alcohol Eobs (kJ/mol) High pressure Low
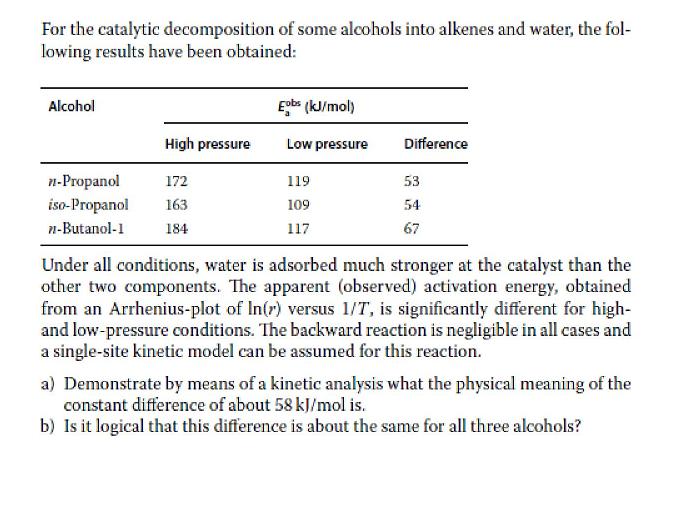
For the catalytic decomposition of some alcohols into alkenes and water, the fol- lowing results have been obtained: Alcohol Eobs (kJ/mol) High pressure Low pressure Difference n-Propanol 172 119 53 iso-Propanol 163 109 54 n-Butanol-1 184 117 67 Under all conditions, water is adsorbed much stronger at the catalyst than the other two components. The apparent (observed) activation energy, obtained from an Arrhenius-plot of In(r) versus 1/T, is significantly different for high- and low-pressure conditions. The backward reaction is negligible in all cases and a single-site kinetic model can be assumed for this reaction. a) Demonstrate by means of a kinetic analysis what the physical meaning of the constant difference of about 58 kJ/mol is. b) Is it logical that this difference is about the same for all three alcohols?
Step by Step Solution
3.45 Rating (164 Votes )
There are 3 Steps involved in it
a The physical meaning of the constant difference of ... View full answer

Get step-by-step solutions from verified subject matter experts


