Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Determine the initial concentrations of S2082- and I- in each of the trials. Recall that Table 1 gives the concentrations of the stock

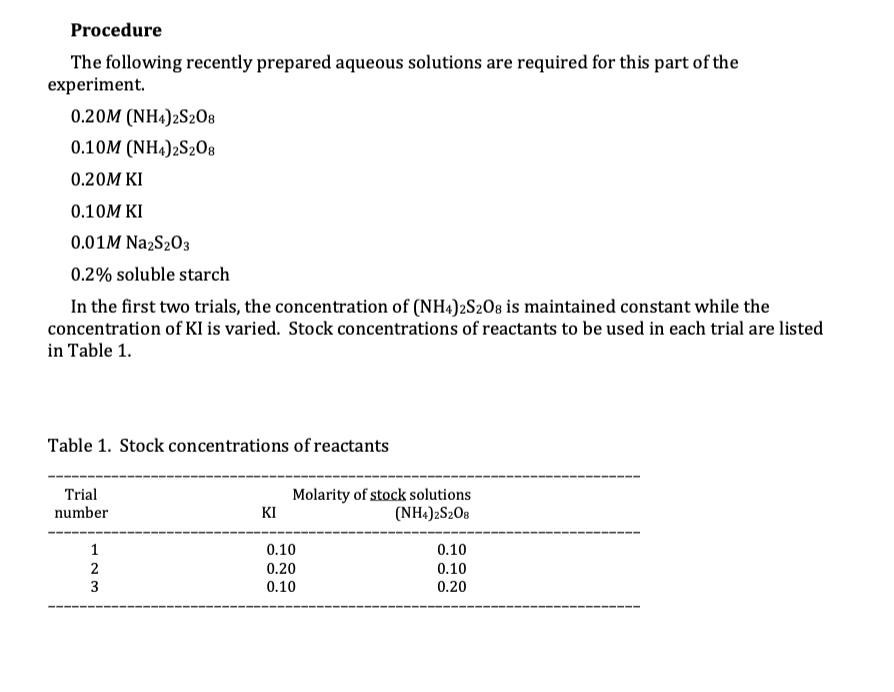
4. Determine the initial concentrations of S2082- and I- in each of the trials. Recall that Table 1 gives the concentrations of the stock solutions that will be used to create the reaction mixtures. The volumes of each reagent used are in the text below that table; the total volume of each reaction mixture will be 65 ml. Provide these initial concentrations in a well-organized, table (either hand-written or electronic). Be sure to indicate units where appropriate and to use appropriate significant figures. Procedure The following recently prepared aqueous solutions are required for this part of the experiment. 0.20M (NH4)2S208 0.10M (NH4)2S2O8 0.20M KI 0.10M KI 0.01M Na2S203 0.2% soluble starch In the first two trials, the concentration of (NH4)2S2O8 is maintained constant while the concentration of KI is varied. Stock concentrations of reactants to be used in each trial are listed in Table 1. Table 1. Stock concentrations of reactants Trial number 1 2 23 KI Molarity of stock solutions (NH4)zSzOs 0.10 0.20 0.10 0.10 0.10 0.20
Step by Step Solution
★★★★★
3.32 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
1 The Procedure for Preparing the Solutions a NH42S2O8 To prepare the solutions first measure out the appropriate amount of NH42S2O8 for each trial Fo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


