Question: Draw the condensed or line-angle structural formula for the aldehyde or ketone produced when each of the following alcohols is oxidized [O]: Spell out the
Draw the condensed or line-angle structural formula for the aldehyde or ketone produced when each of the following alcohols is oxidized [O]: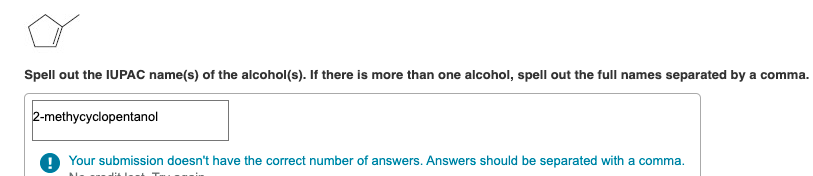
![produced when each of the following alcohols is oxidized [O]: Spell out](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f848ee500e2_62966f848ede1da0.jpg)
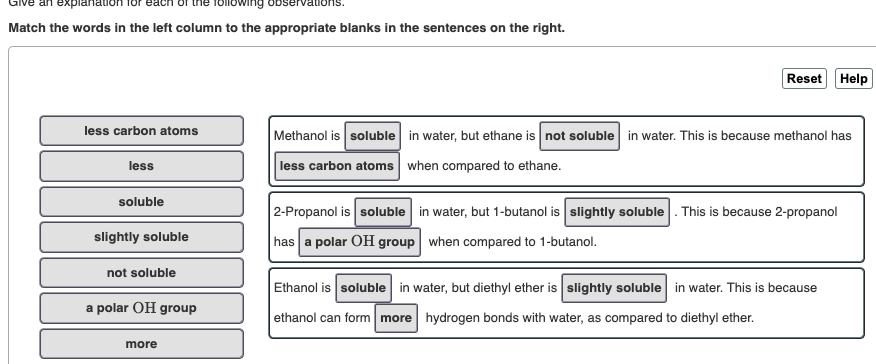
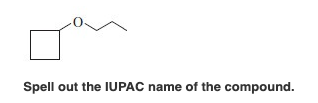
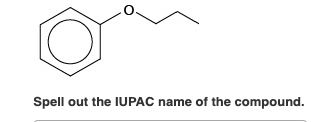





Spell out the IUPAC name(s) of the alcohol(s). If there is more than one alcohol, spell out the full names separated by a comma. 2-methycyclopentanol O Your submission doesn't have the correct number of answers. Answers should be separated with a comma. Spell out the IUPAC name(s) of the alcohol(s). If there is more than one alcohol, spell out the full names separated by a comma. butanol Submit Previous Answers Request Answer * Incorrect; Try Again; 4 attempts remaining - Part B CH3 CH3 -CH2-C=CH-CH3 Spell out the IUPAC name(s) of the alcohol(s). If there is more than one alcohol, spell out the full names separated by a comma. 3-methylpent-2-ene, 3-methylpent ing Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help less carbon atoms Methanol is soluble in water, but ethane is not soluble in water. This is because methanol has less carbon atoms when compared to ethane. less soluble 2-Propanol is soluble in water, but 1-butanol is slightly soluble. This is because 2-propanol has a polar OH group when compared to 1-butanol. slightly soluble not soluble a polar OH group Ethanol is soluble in water, but diethyl ether is slightly soluble in water. This is because ethanol can form more hydrogen bonds with water, as compared to diethyl ether. more Spell out the IUPAC name of the compound. Spell out the IUPAC name of the compound. CH3 CH3-CH-CH2-CH2-OH Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. OH Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. OH CH3 -CH2-CH-CH3 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default. CH3 -CH2-CH2-CH2-CH2-OH Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


