Question: example how it need to be done. thanks please read it. there is no data. just follow the intructions. thats all i have thx. i


 example how it need to be done. thanks
example how it need to be done. thanks 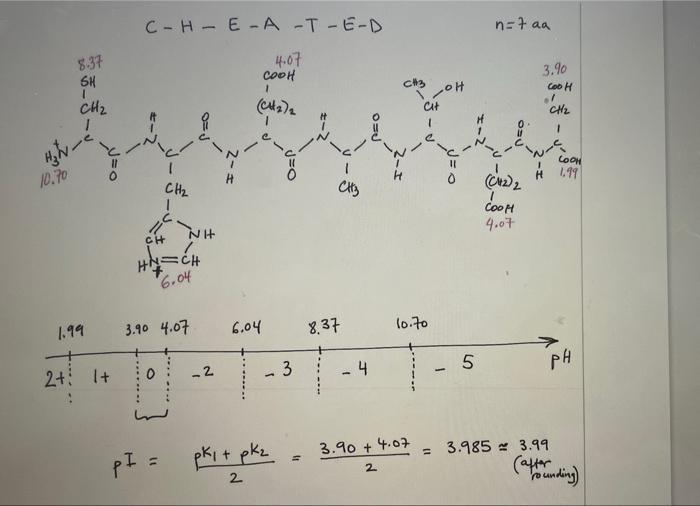
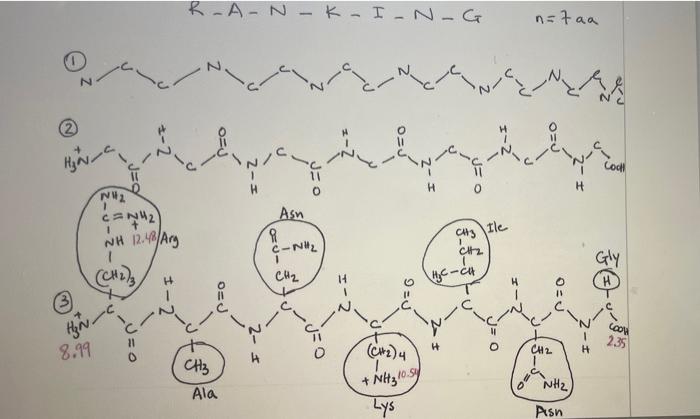
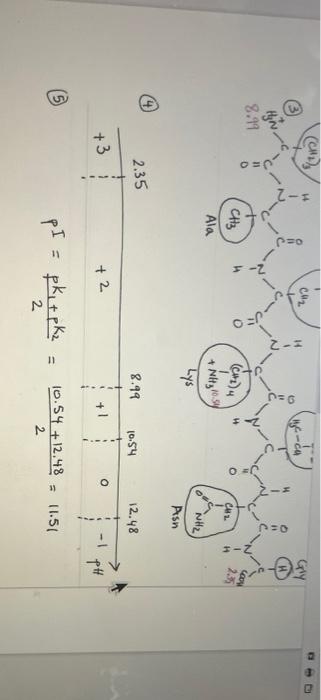
Worksheet on how to draw the structure of a peptide and calculate its isoelectric point Please follow the instructions below and the examples attached to learn about drawing a peptide and calculating its isoelectric point. There are 5 major steps to do so: 1. Start by counting how many amino-acids are there in the peptide. The backbone of the protein is made of N-C-C repeats that are equal to the number of amino acids in the peptide. For a peptide that has 5 amino acids, you should connect 5 N-C-C repeats. The backbone should be drawn in zig zag (see example 1 step 1) to mimic as close as possible the geometric angles for each atom (109.5' for Ca and for N, and 120 for the C of the carbonyl group). Drawing the backbone in zig zag allows you to stagger the R groups (side chains) on either side of the backbone for more clarity. See below. 2- Then, start to fill in hydrogens and oxygens on the protein backbone. Each N-C-C repeat constitutes the amino group (N), the first carbon is Ca, and the second C is that of the carbonyl group. The amide bond exists between the C of the carbonyl group from one N- C-C repeat and the N from the amino group of the N-C-C repeat that immediately follows it. To be able to calculate the pl, you need to draw everything in their fully protonated version. See step 2 in example 1 for more help. a. The N of first N-C-C repeat is the N-terminus and should start with NH3. b. The last carbon in the chain is the C-terminus and should be labeled COOH. You can either add a H to each Ca or leave it intact for more clarity. If you do so, C. You can either add a H to each Ca or leave it intact for more clarity. If you do so, it is assumed that the H is there. d. Add a double bond (=) to the second carbon of each N-C-C repeat to take care of all the carbonyl groups. e. Add the amide hydrogens (-H) to the N of each repeat (except the first because it has already been labeled NH3). 3. Add the side chains that correspond to each amino-acid on the Ca of each repeat (that is the first carbon of each repeat. The second carbon of each repeat should have already been labeled with =0 in step 2 above). See step 3 in example 1. Look up the pka values (see you book, Manz page 5) for each of the following: a. The N-terminus amino group: the pka of this group should be that of the amino group of the first amino acid in the sequence (that is Arg in example 1 and Cys in example 2) b. The C-terminus carboxylic group: the pka of this group should be that of the carboxylic group of the last amino acid in the sequence (that is Gly in example 1 and Asp in example 2) c. All other pka values that correspond to ionizable side chains (Arg and Lys in example 1, and Cys, His, Asp, and Glu in example 2) a 4- Rank the pKa values by increasing order on a pH scale (see step 4 in example 1). Count the number of positive charges on the entire peptide and write that number below the first pKa value on the scale. As you go above the first pka value, you start deprotonating the entity to which this pKa value corresponds. This is most often the carboxylic group at the N-terminus. You have to recalculate the total net charge after each pka value, or more accurately, in each pH range. 5. To calculate the pl, choose the two pka values that surround the net charge of 0 and average them. See example 1 step 5. Keep in mind that the net charge does not always increase in increments of one. This usually occurs when you have the same amino acid repeated more than once in the sequence. For instance, in example 2, the amino acid Glu is repeated twice. When the pH increases above 4.07 (which is the pka of Glu's side chain), both COOH groups in both Glu amino acids get deprotonated at the same time and the net charge of the molecule decreases from 0 to -2. C-HE-A-T-E-D n=faa 837 4.07 3.90 SH COOH 1 (CH)2 CH3 TOH CH Cir COOH . CN2 1 PEO Pro 2- COOH O=P 10.70 (cm, 4 1.99 ) H CH CH3 Cool I NH 4.07 CH Hly=CH 6.04 1.99 3.90 4.07 6.04 8.37 10.70 3 5 0 4 PH -2 2+ lt pki + pkz pI = 11 3.90 +4.07 2 = 3.985 3.99 (after rounding) 2 R-AN-K - ING n=faa N Ha a Cod H 1 1 N2 CEN2 NH 12. 48/Arg (CH2) CH3 Ile Asn C-NH2 1 CH CH2 HC-ch H I-2 DEJ OS t@u- coord Hinc 8.99 2.35 11 0 I-2 (CH2)4 CH2 H CH 10.SK + NH3 NH2 Ala Lys Asn E 14 B2 CH -ca OED = Hyn 8.99 CH3 (CH4 CH2 235 H Ala + Lys * Noltyrosy NH Asn 2.35 8.99 10.54 12.48 o +3 + 1 + 2 -1 PH pI = 1) pk + pk2 10.54 + 12.48 2 = 11:51 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


