Question: Here are two ring closing electrocyclic reactions. The wavy line represents a bond whose position in space has yet to be determined (up or down)
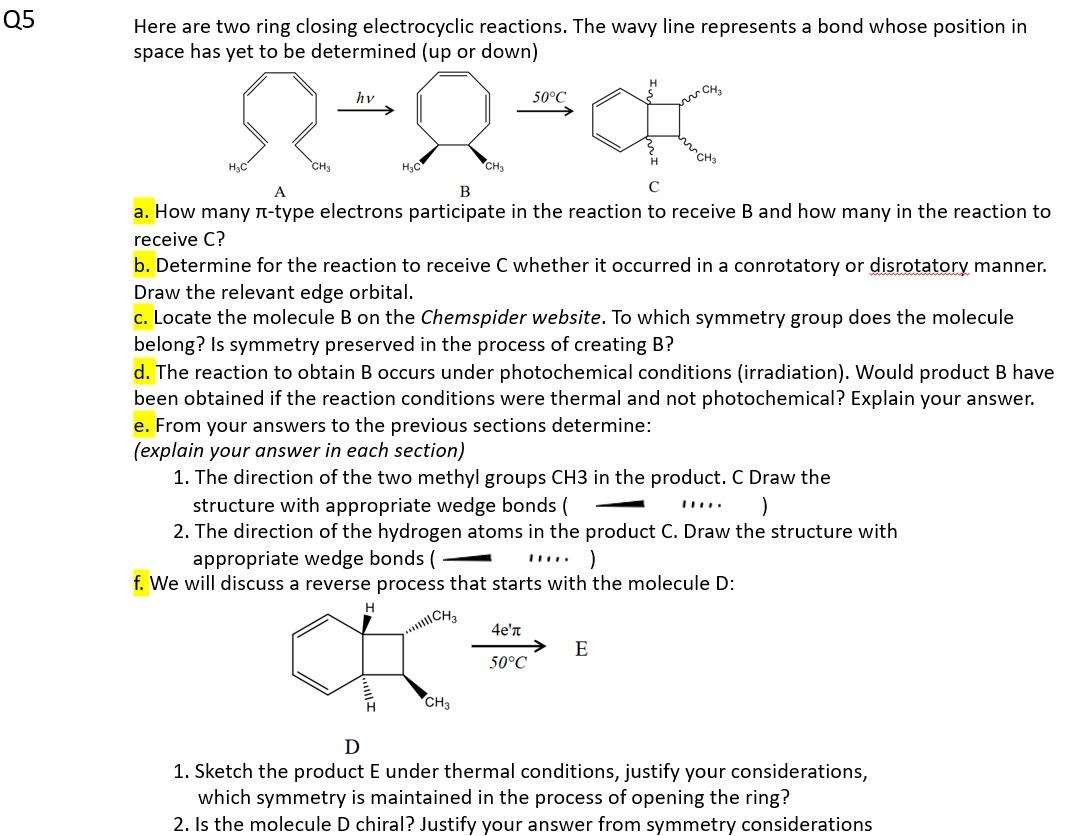
Here are two ring closing electrocyclic reactions. The wavy line represents a bond whose position in space has yet to be determined (up or down) a. How many -type electrons participate in the reaction to receive B and how many in the reaction to receive C ? b. Determine for the reaction to receive C whether it occurred in a conrotatory or disrotatory manner. Draw the relevant edge orbital. c. Locate the molecule B on the Chemspider website. To which symmetry group does the molecule belong? Is symmetry preserved in the process of creating B? d. The reaction to obtain B occurs under photochemical conditions (irradiation). Would product B have been obtained if the reaction conditions were thermal and not photochemical? Explain your answer. e. From your answers to the previous sections determine: (explain your answer in each section) 1. The direction of the two methyl groups CH3 in the product. C Draw the structure with appropriate wedge bonds ( ) 2. The direction of the hydrogen atoms in the product C. Draw the structure with appropriate wedge bonds ( ) f. We will discuss a reverse process that starts with the molecule D: D 1. Sketch the product E under thermal conditions, justify your considerations, which symmetry is maintained in the process of opening the ring? 2. Is the molecule D chiral? Justify your answer from symmetry considerations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


