Question: I attached all the necessary information needed to answer the questions. Can someone please answer the questions I would appreciate it. They're on the last
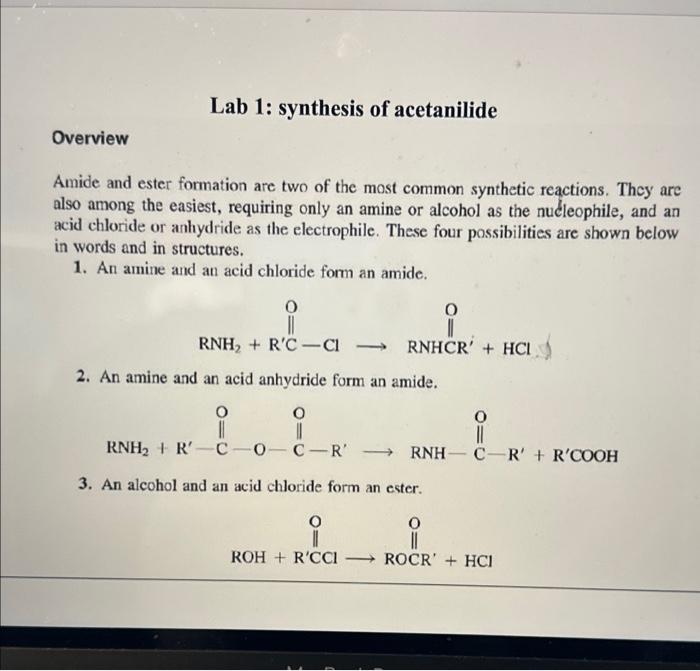

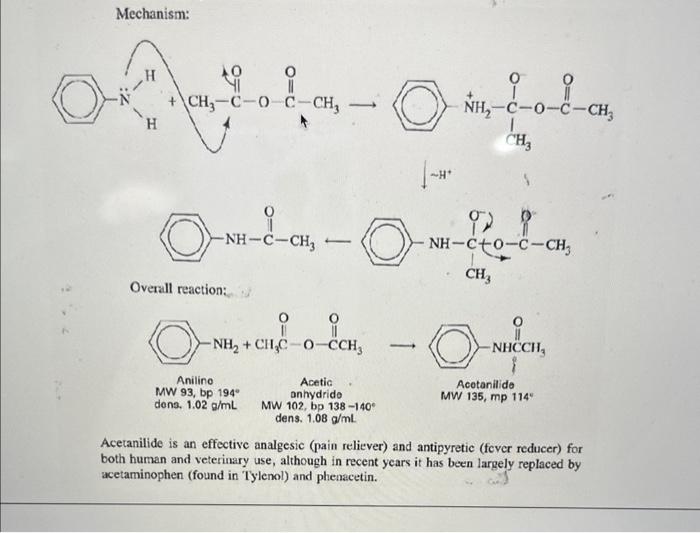
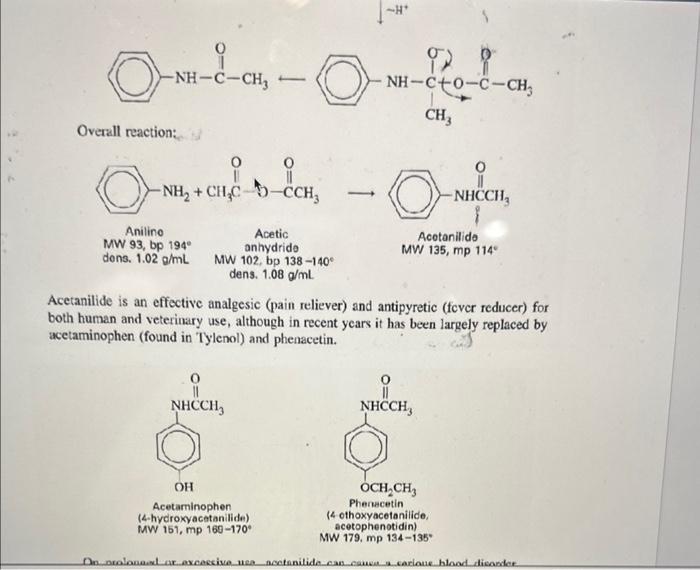
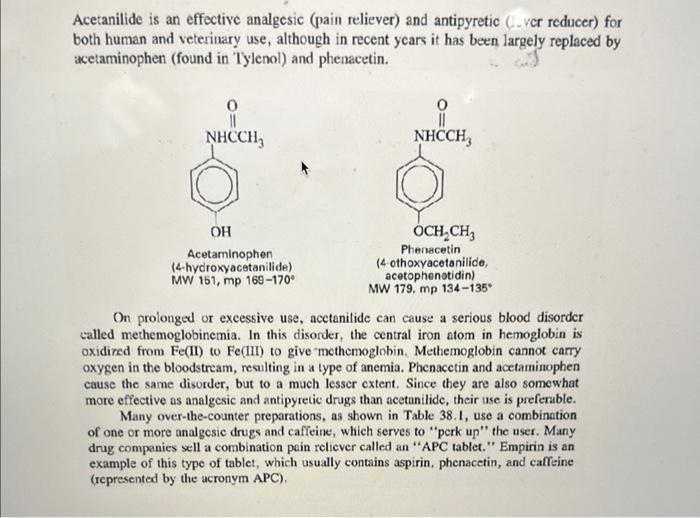
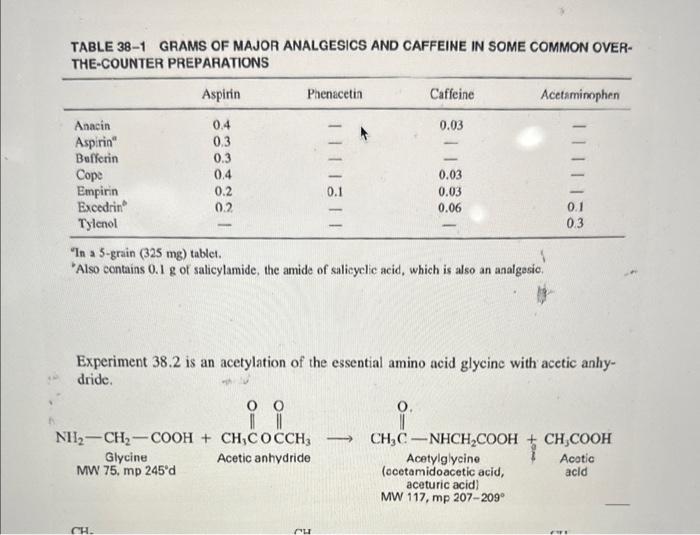
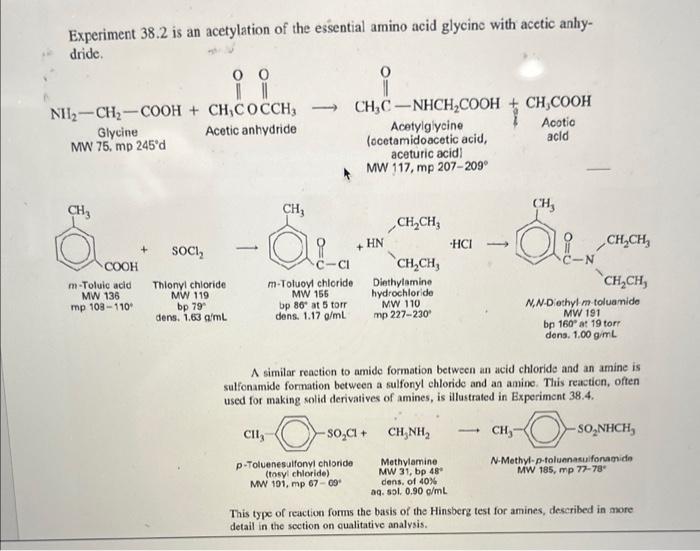


Overview Amide and ester formation are two of the most common synthetic reactions. They are also among the easiest, requiring only an amine or alcohol as the nucleophile, and an acid chloride or anhydride as the electrophile. These four possibilities are shown below in words and in structures. 1. An amine and an acid chloride form an amide. 2. An amine and an acid anhydride form an amide. 3. An alcohol and an acid chloride form an ester. RNH2+RCClRNHCR+HCl 2. An amine and an acid anhydride form an amide. 3. An alcohol and an acid chloride form an ester. 4. An alcohol and an acid anhydride form an ester. The mechanism is that the unshared pair on the nucleophile attacks the positive carbonyl carbon leading to displacement of a leaving group ( Cl or OCR). In Experiment 38.1 you will use aniline and acctic anhydride to form an amide. Mechanism: fH+ Overall reaction: Acotanilido MW 135, mp 114" Acetanilide is an effective analgesic (pain reliever) and antipyretic (fever reducer) for both human and veterinary use, although in recent years it has been largely replaced by acetaminophen (found in Tylenol) and phenacetin. Overall reaction: Acetanilide is an effective analgesic (pain reliever) and antipyretic (fever reducer) for both human and veterinary use, although in recent years it has been largely replaced by acetaminophen (found in Tylenol) and phenacetin. Acetanilide is an effective analgesic (pain reliever) and antipyretic (L ver reducer) for both human and veterinary use, although in recent years it has been largely replaced by acetaminophen (found in Tylenol) and phenacetin. On prolonged or excessive use, acctanilide can cause a serious blood disorder called methemoglobinemia. In this disorder, the central iron atom in hemoglobin is oxidired from Fe(II) to Fe(III) to give methemoglobin. Methemoglobin cannot carry oxygen in the bloodstream, resulting in a type of anemia. Phenacetin and acetaminophen cause the same disorder, but to a much lesser extent. Since they are also somewhat more effective as analgesic and antipyretic drugs than acetanilide, their use is preferable. Many over-the-counter preparations, as shown in Table 38.1, use a combination of one or more analgesic drugs and caffeine, which serves to "perk up" the user. Many drug companies sell a combination pein relicver called an "APC tablet." Empirin is an example of this type of tablet, which usually contains aspirin, phenacetin, and cafteine (represented by the acronym APC). TABLE 38-1 GRAMS OF MAJOR ANALGESICS AND CAFFEINE IN SOME COMMON OVERTHE-COUNTER PREPARATIONS "In a 5-grain (325 mg) tablet. 'Also contains 0.1g of salicylamide, the amide of salicyclic acid, which is also an analgesic. Experiment 38.2 is an acetylation of the essential amino acid glycine with acetic anhydride. Experiment 38.2 is an acetylation of the essential amino acid glycine with acetic anhydride. MW 75,mp245d Acetic anhydride Acetylglycine locetamidoacetic acid, aceturic acid MW 117, mp 207-209 N,N-Dothyl m toluanido MW 191 bp 160 at 19 tor A similar reaction to amide formation between an acid chloride and an amine is sulfonamide formation between a sulfonyl chloride and an amine. This reacticn, often used for making solid derivatives of amines, is illustrated in Experiment 38.4. CH3SO2Cl+CH3NH2CH3SO2NHCH3 This type of reaction forms the basis of the Hinsberg test for amines, described in more detail in the section on cualitative analysis. Protocol: ACETANILIDE FROM ANILINE AND ACETIC ANHYDRIDE Prelab 1. Calculate the volume of 10mmol of aniline. 2. Calculate the theoretical yield of acetanilide. Special Hazards Aniline is highly toxic on ingestion and is a suspected carcinogen on long-term exposure to high concentrations. Avoid breathing its vapors or contact with skin. Use of gloves is recommended; if skin contact occurs, wash thoroughly with soap. When mixing acid and water, always add acid to water, not vice versa. Procedure Dissolve aniline ( mL,10mmol) in a mixture of water (25mL) and conc. HCl (0.8mL,10mmol). Measure out acetic anhydride (1.2mL,12mmol) and prepare a solution of sodium acetate (1.0g of anhydrous NaOAc,12mmol) in water (5mL). While stirring, add the acetic anhydride to the solution of aniline hydrochloride and immediately add the sodium acetate solution. The acetate ion serves to deprotonate the anilinium ion so that the aniline can act as a nucleophile and attack the acetic anhydride. Cool the crude mixture in an ice bath and collect the product. You will save this product for your next 1) Draw out the mechanism for today's synthesis - name all products and reactants. Give the hybridization of all atoms in both products and reactants. 2) What is the nucleophile in this reaction? Protonated aniline (anilinium) has a pKa of about 5. Based on this property, how well would your reaction today run at pH4.0 ? How about 6.0 ? 3) Describe the synthesis of each of the following using an appropriate starting reagent (name and structure) and acetic anhydride: - N-methyl acetamide - Cyclopentyl ethanoate - Cyclohexyl formamide - N,N-diethyl 2-methyl-propamide 4) Draw out three correct Lewis resonance structures for aniline. Based on these three structures, draw the "rhinoceros" that represents a realistic total picture of the molecule. 5) Imagine you were an electrophillic atom (an atom in need of electrons). Where on aniline would you most likely find extra electrons? (hint: look at the previous problem). 6) Based on #4, predict the product of aniline and sulfur trioxide (the sulfur acts as an electrophile) Overview Amide and ester formation are two of the most common synthetic reactions. They are also among the easiest, requiring only an amine or alcohol as the nucleophile, and an acid chloride or anhydride as the electrophile. These four possibilities are shown below in words and in structures. 1. An amine and an acid chloride form an amide. 2. An amine and an acid anhydride form an amide. 3. An alcohol and an acid chloride form an ester. RNH2+RCClRNHCR+HCl 2. An amine and an acid anhydride form an amide. 3. An alcohol and an acid chloride form an ester. 4. An alcohol and an acid anhydride form an ester. The mechanism is that the unshared pair on the nucleophile attacks the positive carbonyl carbon leading to displacement of a leaving group ( Cl or OCR). In Experiment 38.1 you will use aniline and acctic anhydride to form an amide. Mechanism: fH+ Overall reaction: Acotanilido MW 135, mp 114" Acetanilide is an effective analgesic (pain reliever) and antipyretic (fever reducer) for both human and veterinary use, although in recent years it has been largely replaced by acetaminophen (found in Tylenol) and phenacetin. Overall reaction: Acetanilide is an effective analgesic (pain reliever) and antipyretic (fever reducer) for both human and veterinary use, although in recent years it has been largely replaced by acetaminophen (found in Tylenol) and phenacetin. Acetanilide is an effective analgesic (pain reliever) and antipyretic (L ver reducer) for both human and veterinary use, although in recent years it has been largely replaced by acetaminophen (found in Tylenol) and phenacetin. On prolonged or excessive use, acctanilide can cause a serious blood disorder called methemoglobinemia. In this disorder, the central iron atom in hemoglobin is oxidired from Fe(II) to Fe(III) to give methemoglobin. Methemoglobin cannot carry oxygen in the bloodstream, resulting in a type of anemia. Phenacetin and acetaminophen cause the same disorder, but to a much lesser extent. Since they are also somewhat more effective as analgesic and antipyretic drugs than acetanilide, their use is preferable. Many over-the-counter preparations, as shown in Table 38.1, use a combination of one or more analgesic drugs and caffeine, which serves to "perk up" the user. Many drug companies sell a combination pein relicver called an "APC tablet." Empirin is an example of this type of tablet, which usually contains aspirin, phenacetin, and cafteine (represented by the acronym APC). TABLE 38-1 GRAMS OF MAJOR ANALGESICS AND CAFFEINE IN SOME COMMON OVERTHE-COUNTER PREPARATIONS "In a 5-grain (325 mg) tablet. 'Also contains 0.1g of salicylamide, the amide of salicyclic acid, which is also an analgesic. Experiment 38.2 is an acetylation of the essential amino acid glycine with acetic anhydride. Experiment 38.2 is an acetylation of the essential amino acid glycine with acetic anhydride. MW 75,mp245d Acetic anhydride Acetylglycine locetamidoacetic acid, aceturic acid MW 117, mp 207-209 N,N-Dothyl m toluanido MW 191 bp 160 at 19 tor A similar reaction to amide formation between an acid chloride and an amine is sulfonamide formation between a sulfonyl chloride and an amine. This reacticn, often used for making solid derivatives of amines, is illustrated in Experiment 38.4. CH3SO2Cl+CH3NH2CH3SO2NHCH3 This type of reaction forms the basis of the Hinsberg test for amines, described in more detail in the section on cualitative analysis. Protocol: ACETANILIDE FROM ANILINE AND ACETIC ANHYDRIDE Prelab 1. Calculate the volume of 10mmol of aniline. 2. Calculate the theoretical yield of acetanilide. Special Hazards Aniline is highly toxic on ingestion and is a suspected carcinogen on long-term exposure to high concentrations. Avoid breathing its vapors or contact with skin. Use of gloves is recommended; if skin contact occurs, wash thoroughly with soap. When mixing acid and water, always add acid to water, not vice versa. Procedure Dissolve aniline ( mL,10mmol) in a mixture of water (25mL) and conc. HCl (0.8mL,10mmol). Measure out acetic anhydride (1.2mL,12mmol) and prepare a solution of sodium acetate (1.0g of anhydrous NaOAc,12mmol) in water (5mL). While stirring, add the acetic anhydride to the solution of aniline hydrochloride and immediately add the sodium acetate solution. The acetate ion serves to deprotonate the anilinium ion so that the aniline can act as a nucleophile and attack the acetic anhydride. Cool the crude mixture in an ice bath and collect the product. You will save this product for your next 1) Draw out the mechanism for today's synthesis - name all products and reactants. Give the hybridization of all atoms in both products and reactants. 2) What is the nucleophile in this reaction? Protonated aniline (anilinium) has a pKa of about 5. Based on this property, how well would your reaction today run at pH4.0 ? How about 6.0 ? 3) Describe the synthesis of each of the following using an appropriate starting reagent (name and structure) and acetic anhydride: - N-methyl acetamide - Cyclopentyl ethanoate - Cyclohexyl formamide - N,N-diethyl 2-methyl-propamide 4) Draw out three correct Lewis resonance structures for aniline. Based on these three structures, draw the "rhinoceros" that represents a realistic total picture of the molecule. 5) Imagine you were an electrophillic atom (an atom in need of electrons). Where on aniline would you most likely find extra electrons? (hint: look at the previous problem). 6) Based on #4, predict the product of aniline and sulfur trioxide (the sulfur acts as an electrophile)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


