Question: I have uploaded a flow chart which represents part A of this worksheet that should be used in answering these questions. I really hope it's
I have uploaded a flow chart which represents part A of this worksheet that should be used in answering these questions. I really hope it's possible that the expert that choose this question can view my previous submitted question. It shows the flow chart that was submitted. I appreciate the help you may offer. Thanks in advance.
The images below contains other information that was given to assist in the completion of this work sheet.


Please give me a feedback asap. I appreciate the help you may offer. Thank you
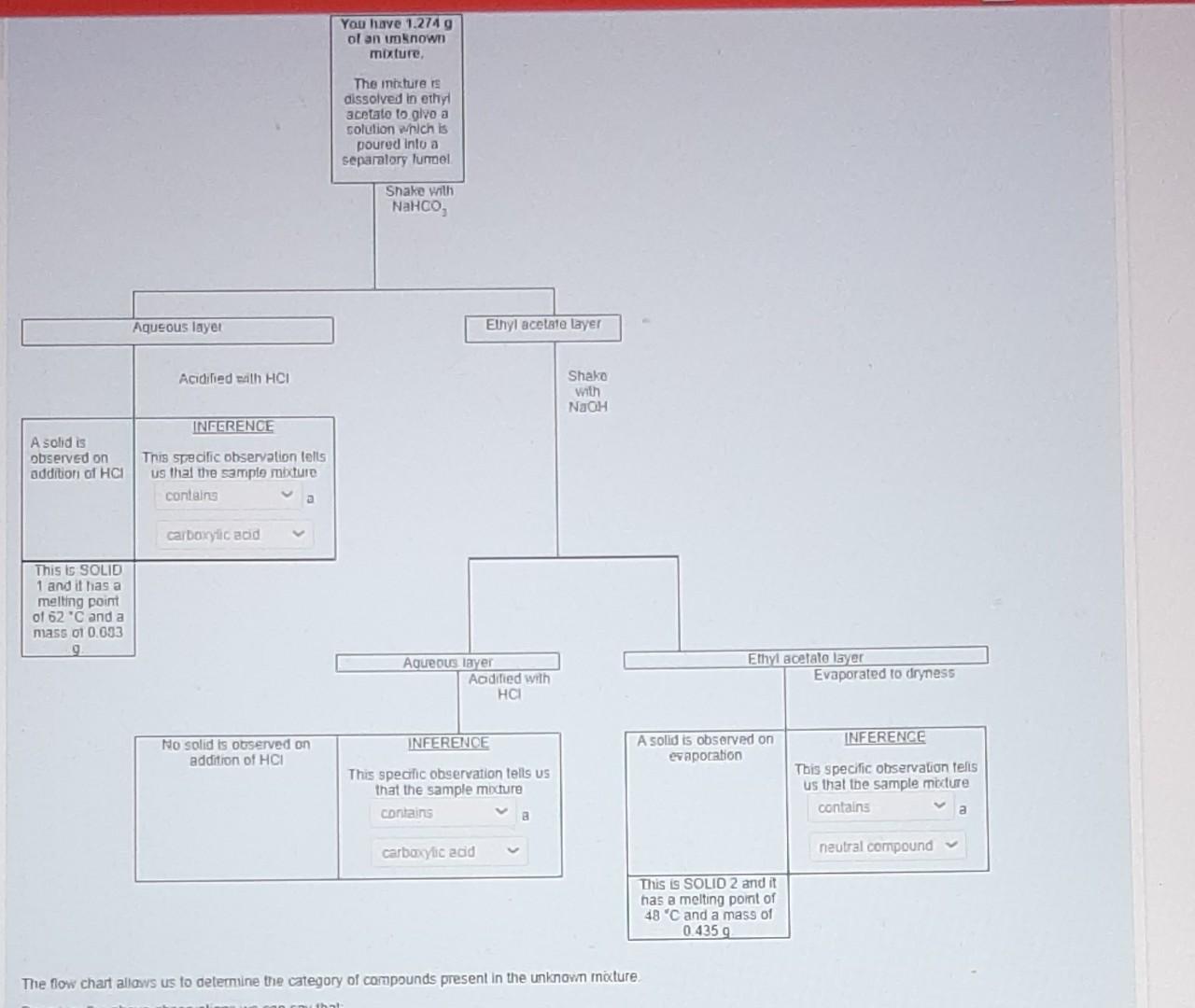
This was an image of the flow chart I submitted previously however I will highlight the area that stated " no solid is observed on the addition of HCL" the answer should have been "does not contain phenol".
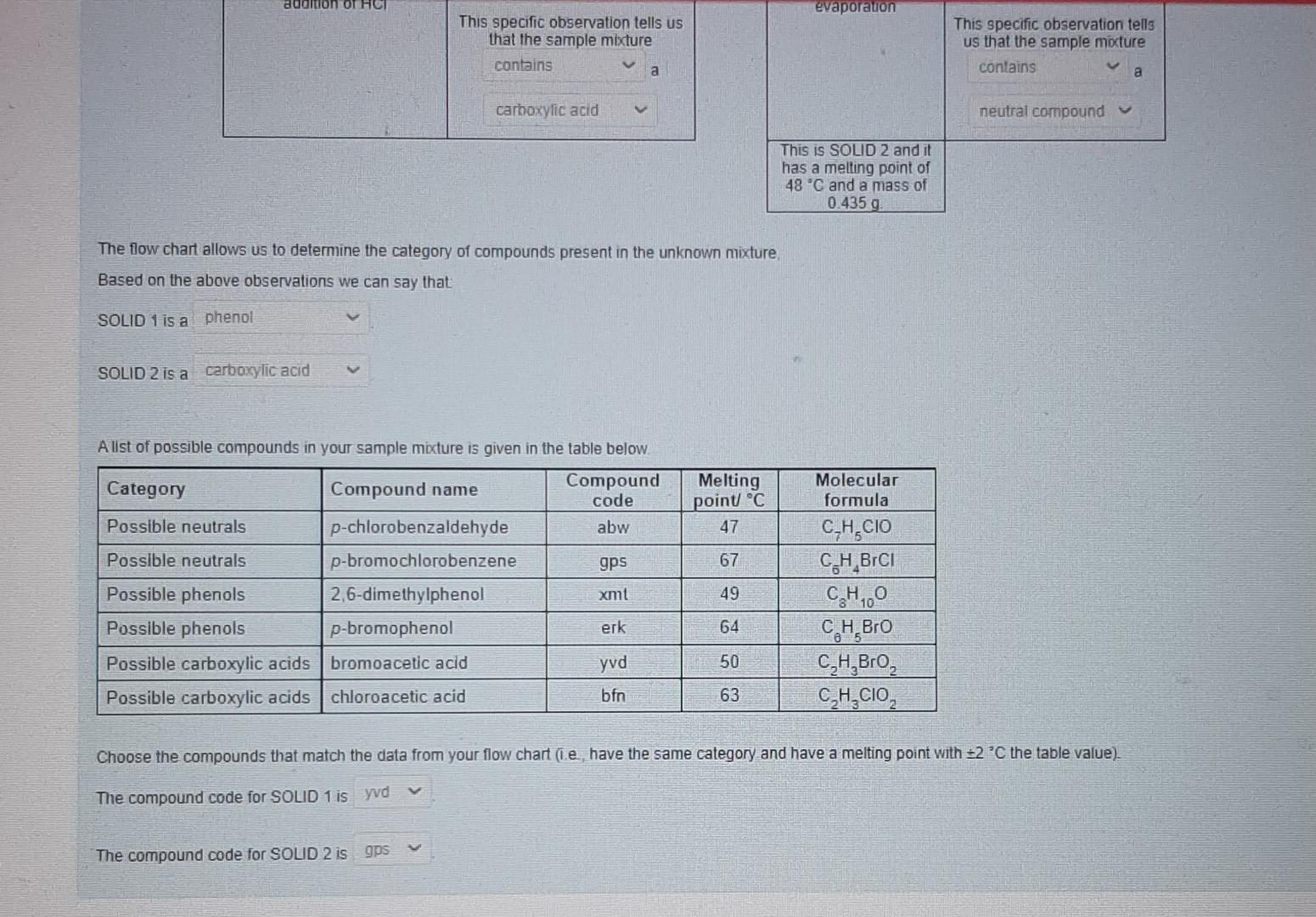
This is an addition of the photo above. However these answers are all incorrect. I will state the correct answers below:
Solid 1 is : carboxylic acid Solid 2 is: a neutral compound
The compound code for SOLID 1 is : bfn The compound code for SOLID 2 is : abw
looking forward to your help. Thank you
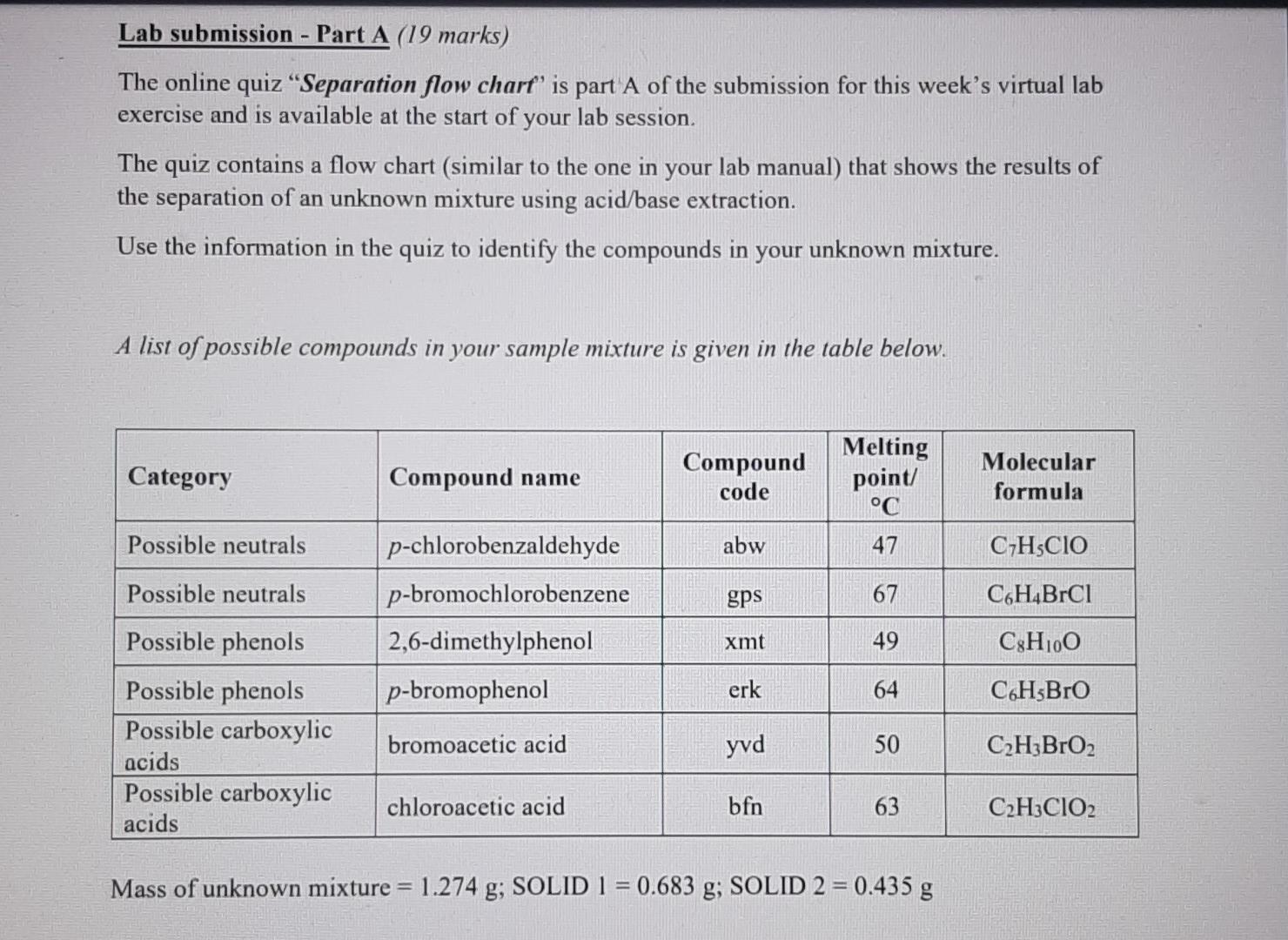
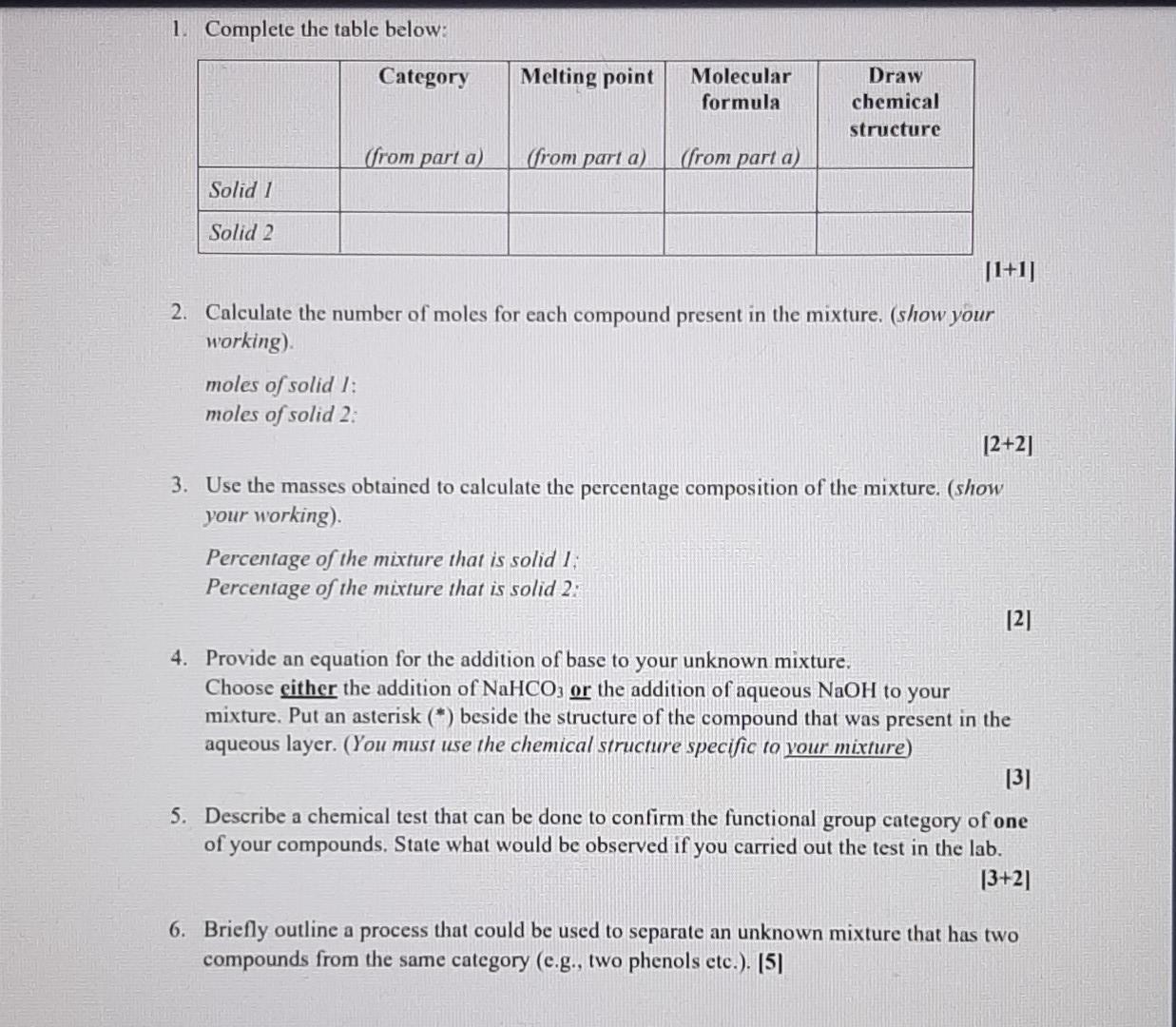
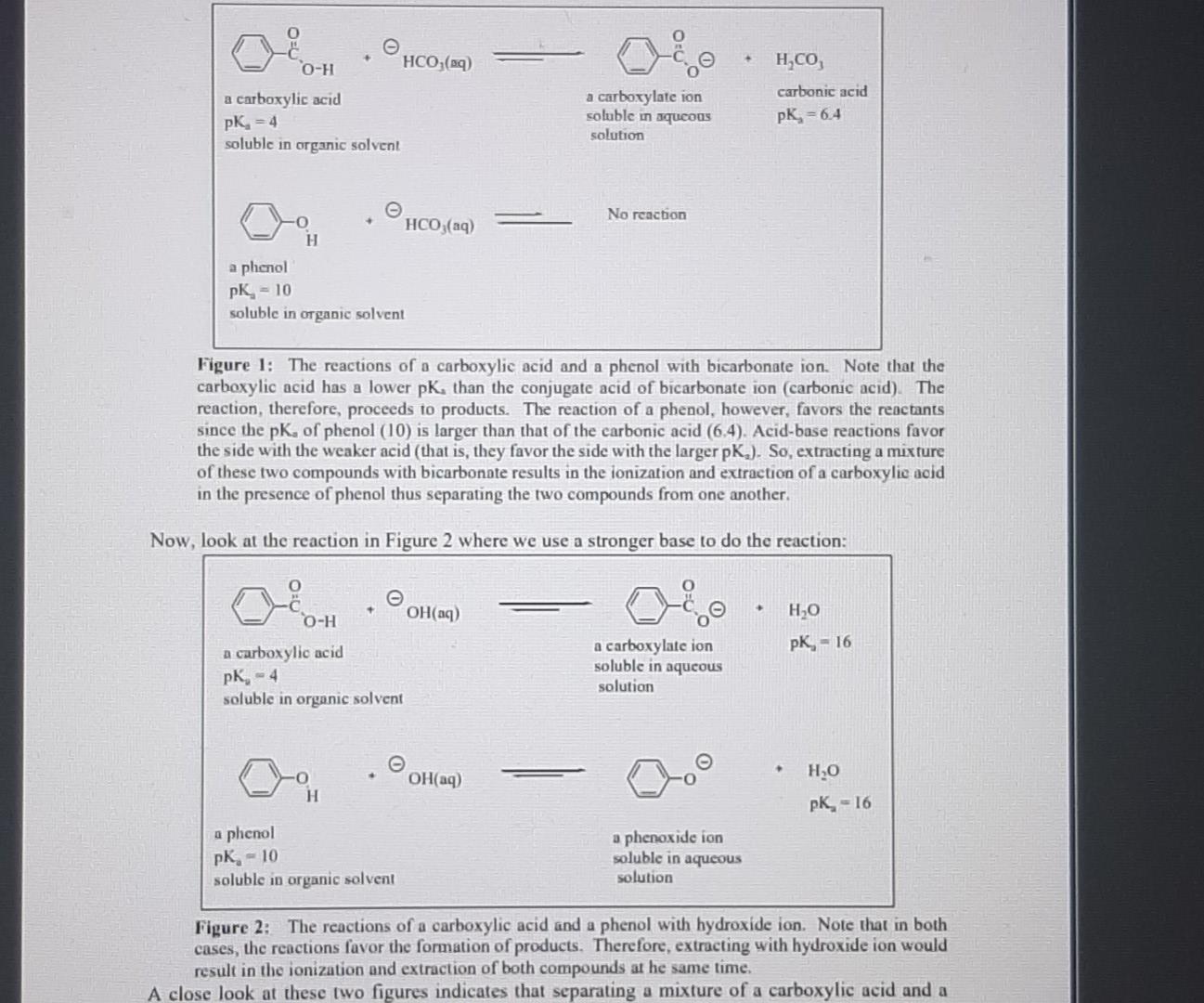
Lab submission - Part A (19 marks) The online quiz Separation flow chart' is part A of the submission for this week's virtual lab exercise and is available at the start of your lab session. The quiz contains a flow chart (similar to the one in your lab manual) that shows the results of the separation of an unknown mixture using acid/base extraction. Use the information in the quiz to identify the compounds in your unknown mixture. A list of possible compounds in your sample mixture is given in the table below. Category Compound name Compound code Melting point/ C 47 Molecular formula Possible neutrals abw CHCIO Possible neutrals gps 67 C6H4BrC1 p-chlorobenzaldehyde p-bromochlorobenzene 2,6-dimethylphenol p-bromophenol xmt 49 C8H100 erk 64 C&H:Bro Possible phenols Possible phenols Possible carboxylic acids Possible carboxylic acids bromoacetic acid yvd 50 C2H3BrO2 chloroacetic acid bfn 63 C2H3CIO2 Mass of unknown mixture = 1.274 g; SOLID 1 = 0.683 g; SOLID 2 = 0.435 g 1. Complete the table below: Category Melting point Molecular formula Draw chemical structure (from part a) (from part a) (from part a) Solid 1 Solid 2 [1+1] 2. Calculate the number of moles for each compound present in the mixture, (show your working) moles of solid 1: moles of solid 2. [2+2] 3. Use the masses obtained to calculate the percentage composition of the mixture. (show your working). Percentage of the mixture that is solid 1 Percentage of the mixture that is solid 2: [2] 4. Provide an equation for the addition of base to your unknown mixture. Choose either the addition of NaHCO3 or the addition of aqueous NaOH to your mixture. Put an asterisk (*) beside the structure of the compound that was present in the aqueous layer. (You must use the chemical structure specific to your mixture) [3] 5. Describe a chemical test that can be done to confirm the functional group category of one of your compounds. State what would be observed if you carried out the test in the lab. [3+21 6. Briefly outline a process that could be used to separate an unknown mixture that has two compounds from the same category (e.g., two phenols etc.). [5] Experimental Aims: The objective of this exercise is to separate a two-component mixture using extraction techniques and then to identify the isolated components by determining their melting points Experimental learning objectives: At the end of this experiment you should be able to: (i) use a separatory/dropping funnel; (ii) dry an organic liquid; (iii) usc a rotary evaporator; (iv) identify the organic phase in an immiscible organic/aqucous mixture; (v) use acid/base reactions to impact the solubility of organic compounds and (vi) determine melting points. Each student will be given a mixture of two substances, which belong to two of the three categories listed below. Possible carboxylic acids Possible phenols Possible neutrals benzoic acid 4-tert-butylphenol 1,4-dimethoxybenzene 2-chlorobenzoic acid 2-naphthol Nuorenc Background Reading: Besides reading the lab manual you will need to do a little bit more. To help you understand the chemical basis of this exercise, you should review Sections 3.5 3.7 in Solomons & Fryhl which concerns the properties of acids and their conjugate bases. Pay particular attention to the use of pk, values. You should also review the appropriate pages in the Mohrig and pay keen attention during your lab talk to acquaint yourself with extraction, washing, drying agents, and recrystallization, Background Information: Extraction is a particularly useful means of separating organic compounds if one compound in the mixture can be chemically converted to an ionic form. The ionic form is soluble in an aqueous layer and can be extracted into it. Other, non-ionic organic compounds in the mixture will remain dissolved in the organic solvent layer. Separation of the two layers results in the separation of the two compounds. The extent to which an acid-base reaction proceeds to completion depends upon the relative acidity of the reactants and products. Reactions occur so that stronger acids and bases are converted into weaker conjugate base and conjugate acids, respectively. The pK, value of the acids gives a measure of the acidity of each compound. Stronger acids have smaller pKa values and their conjugate bases are weaker. The position of an acid-base equilibrium can then be predicted from knowledge of the pk, values of the acids involved. Take a look at the following acid-base reactions in Figure 1, paying attention to the position of the equilibrium and its relationship to the pk, values given. geot O-H HCO3(aq) H,CO, a carboxylic acid pK, -4 soluble in organic solvent a carboxylate ion soluble in aqueous solution carbonic acid pk = 6.4 No reaction HCO3(aq) H a phenol pK, - 10 soluble in organic solvent Figure 1: The reactions of a carboxylic acid and a phenol with bicarbonate ion. Note that the carboxylic acid has a lower pKthan the conjugate acid of bicarbonate ion (carbonic acid). The reaction, therefore, proceeds to products. The reaction of a phenol, however, favors the reactants since the pK, of phenol (10) is larger than that of the carbonic acid (6.4). Acid-base reactions favor the side with the weaker acid (that is, they favor the side with the larger pK.). So, extracting a mixture of these two compounds with bicarbonate results in the ionization and extraction of a carboxylic acid in the presence of phenol thus separating the two compounds from one another. Now, look at the reaction in Figure 2 where we use a stronger base to do the reaction: O-H OH(aq) HO PK, - 16 a carboxylic acid pK, - 4 soluble in organic solvent a carboxylate ion soluble in aqueous solution OH(aq) ,0 H pk = 16 a phenol pK, - 10 soluble in organic solvent a phenoxide ion soluble in aqueous solution Figure 2: The reactions of a carboxylic acid and a phenol with hydroxide ion. Note that in both cases, the reactions favor the formation of products. Therefore, extracting with hydroxide ion would result in the ionization and extraction of both compounds at he same time. A close look at these two figures indicates that separating a mixture of a carboxylic acid and a its conjugate base by bicarbonate. The conjugate base of the carboxylic acid, being an ionic species, is soluble in the aqueous layer while the phenol (left unionized) would remain dissolved in the organic layer. However, if we were to extract with hydroxide ion, both the carboxylic acid and the phenol would be converted into their conjugate bases (see Figure 2). The conjugate bases, again are both ionic species and therefore soluble in the aqueous layer. This means that both compounds would be extracted at the same time, resulting in no separation A neutral compound will not react with either bicarbonate ion or hydroxide ion since * neutral compound does not have protons acidic enough to be removed by these bases. Therefore, such a compound will remain dissolved in the organic layer, no matter which base is added. Por example, a mixture of neutral compound and a carboxylic acid can be separated using bicarbonate ion since only carboxylic acid will be ionized by the bicarbonate ion Once extracted, the carboxylic acid and phenol can both be recovered by adding HCl to the aqueous solutions. The carboxylate ion and phenoxide will both be protonated by HCl, resulting in the formation of the original carboxylic acid and phenol, neither of which is soluble in water so they precipitate from solution. The solid can then be isolated by filtration Figure 3 shows this chemistry for you. Cl(84) HCl(aq) O-H a carboxylate ion soluble in aqueous solution PK, - -7 a carboxylic acid (pK, = 4) precipitates from aqueous solution Ch 00 . pk = -7 HCl(aq) H a phenoxide ion a phenol (pK, = 10) soluble in aqueous precipitates from aqueous solution solution Figure 3: The reactions of a carboxylate ion and a phenoxide ion with HCL Since HCl is a stronger acid than either of the conjugate acids, the products are favored in both cases. The products, a carboxylic acid and a phenol, are insoluble in aqueous solutions and precipitate from solution. The resulting solids can be isolated and their melting points determined You have 1.2749 of an unknown mixture The mixturers dissolved in ethyl acetato lo alvo a solution which is poured into a separatory lurrel Shake with NaHCO, Aqueous layer Ethyl acetate layer Acidified with HCI Shako with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture contains a carboxylic acid This is SOLID 1 and it has a melting point of 62 C and a mass 01 0.633 9 Aqueous layer Aodified with HCI Ethyl acetato layer Evaporated to dryness INFERENCE INFERENCE No solid is observed on addition of HCI A solid is observed on evaporation This specific observation tells us that the sample mature contains 8 This specific observation tells us that the sample micture contains a carboxylic add neutral compound This is SOLID 2 and it has a melting point of 48'C and a mass of 0.4359 The flow chart allows us to determine the category of compounds present in the unknown mocture addition of HCI evaporation This specific observation tells us that the sample mixture contains This specific observation tells us that the sample mocture contains a carboxylic acid neutral compound This is SOLID 2 and it has a melting point of 48C and a mass of 0.435 g The flow chart allows us to determine the category of compounds present in the unknown mixture, Based on the above observations we can say that SOLID 1 is a phenol SOLID 2 is a carboxylic acid Melting point C 47 67 A lst of possible compounds in your sample mixture is given in the table below Category Compound Compound name code Possible neutrals p-chlorobenzaldehyde abw Possible neutrals p-bromochlorobenzene gps Possible phenols 2,6-dimethylphenol xmt Possible phenols p-bromophenol erk Possible carboxylic acids bromoacetic acid Possible carboxylic acids chloroacetic acid bfn 49 Molecular formula C.H.CO C.H.BICI C.4.0 CH.Bro C.H.Broz CHCIO, 64 yud 50 63 Choose the compounds that match the data from your flow chart (e have the same category and have a melting point with 2 C the table value) The compound code for SOLID 1 is yvd The compound code for SOLID 2 is gps
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


