Question: i need to solution as soon as possible and please i am waiting for this answer and give me the answer by typing thank you

i need to solution as soon as possible and please i am waiting for this answer and give me the answer by typing thank you
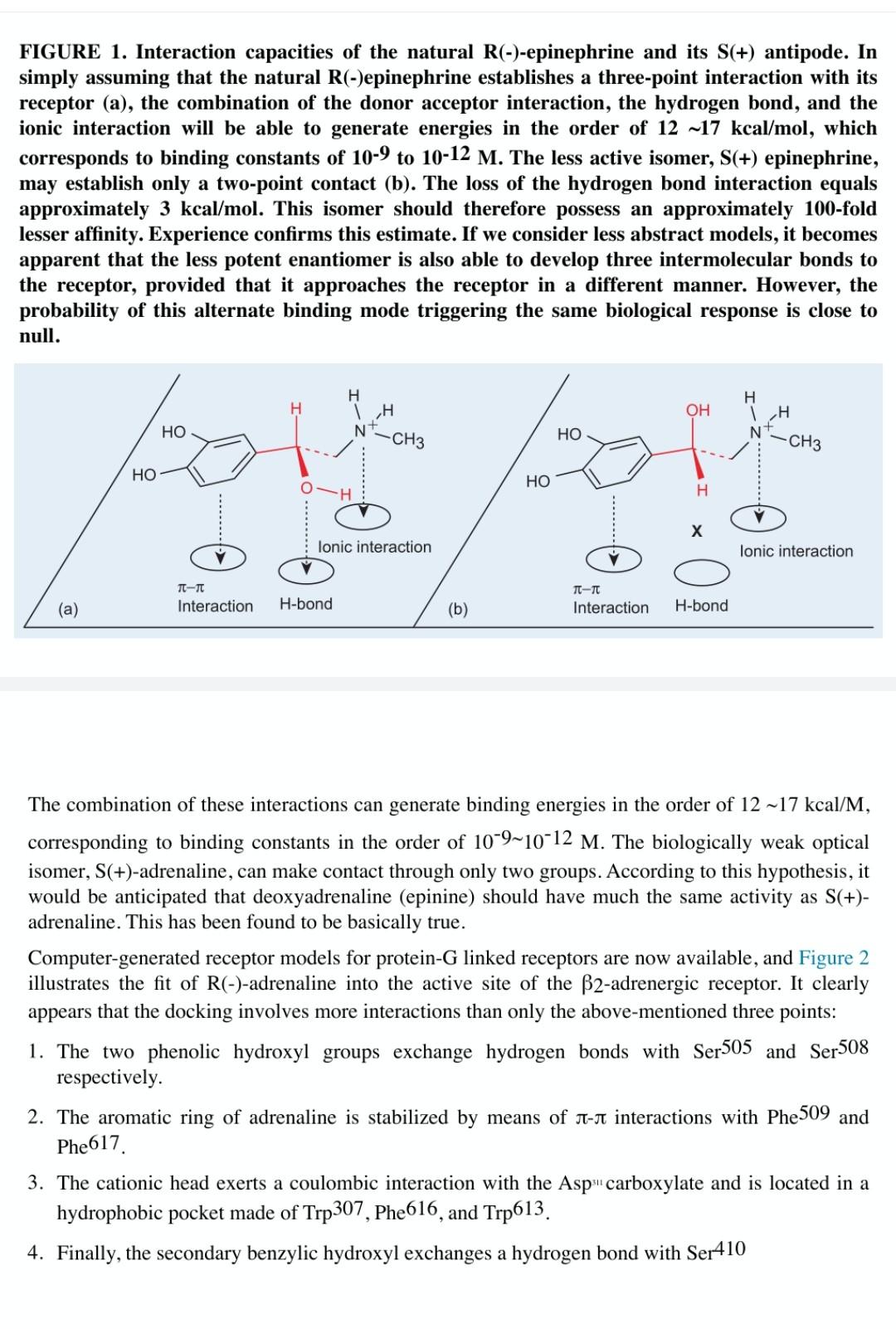
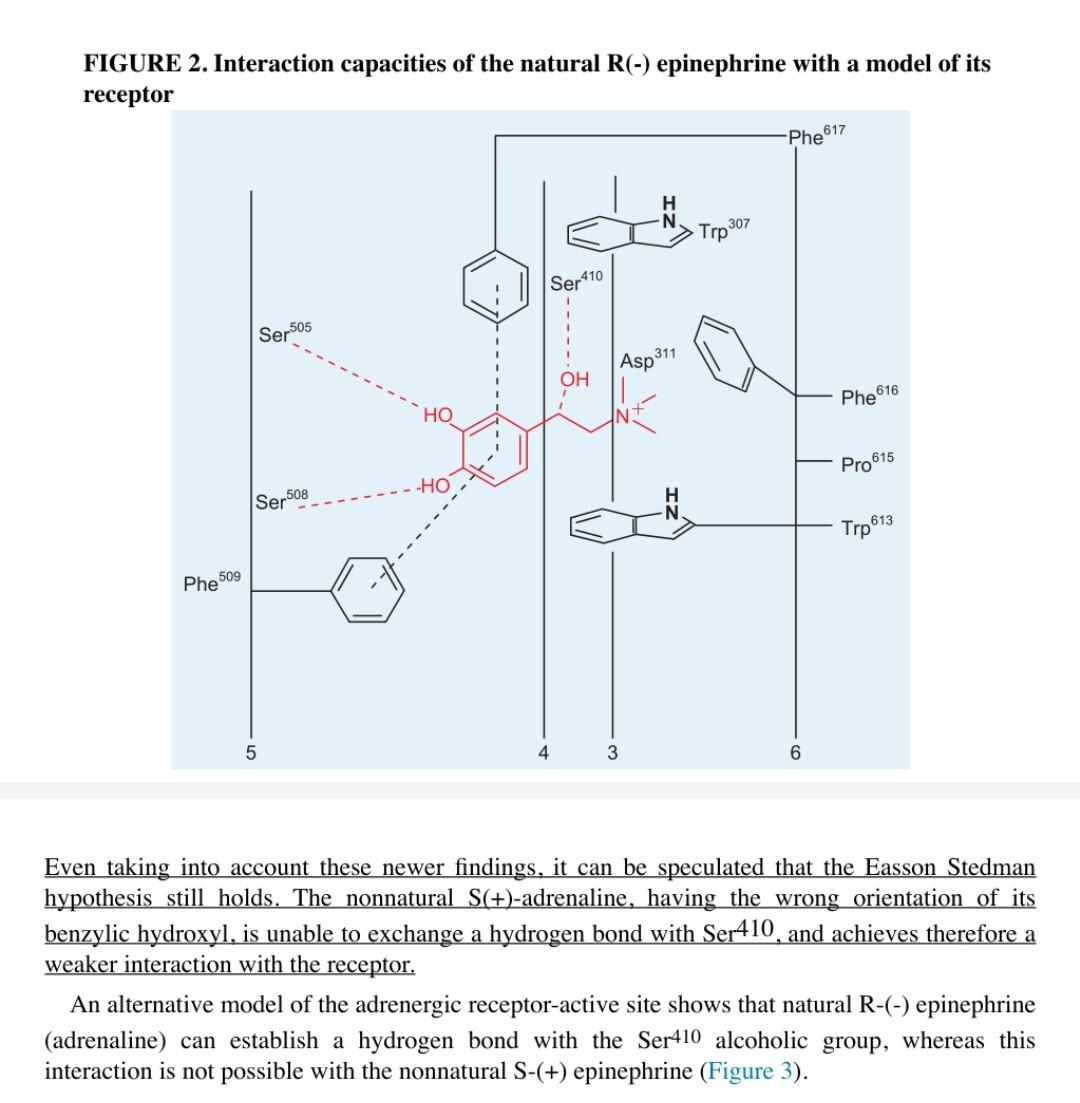
C. Use the three-point contact model to explain Figure 1a and Figure 1b. FIGURE 1. Interaction capacities of the natural R(-)-epinephrine and its S(+) antipode. In simply assuming that the natural R(-)epinephrine establishes a three-point interaction with its receptor (a), the combination of the donor acceptor interaction, the hydrogen bond, and the ionic interaction will be able to generate energies in the order of 12 ~17 kcal/mol, which corresponds to binding constants of 10-9 to 10-12 M. The less active isomer, S(+) epinephrine, may establish only a two-point contact (b). The loss of the hydrogen bond interaction equals approximately 3 kcal/mol. This isomer should therefore possess an approximately 100-fold lesser affinity. Experience confirms this estimate. If we consider less abstract models, it becomes apparent that the less potent enantiomer is also able to develop three intermolecular bonds to the receptor, provided that it approaches the receptor in a different manner. However, the probability of this alternate binding mode triggering the same biological response is close to null. OH LH N H 1 H HO -CH3 HO N -CH3 HO HO H lonic interaction lonic interaction (0) TT-TT Interaction TT- Interaction (a) H-bond (b) H-bond The combination of these interactions can generate binding energies in the order of 12 ~17 kcal/M, corresponding to binding constants in the order of 10-9-10-12 M. The biologically weak optical isomer, S(+)-adrenaline, can make contact through only two groups. According to this hypothesis, it would be anticipated that deoxyadrenaline (epinine) should have much the same activity as S(+)- adrenaline. This has been found to be basically true. Computer-generated receptor models for protein-G linked receptors are now available, and Figure 2 illustrates the fit of R(-)-adrenaline into the active site of the B2-adrenergic receptor. It clearly appears that the docking involves more interactions than only the above-mentioned three points: 1. The two phenolic hydroxyl groups exchange hydrogen bonds with Ser505 and Ser508 respectively. 2. The aromatic ring of adrenaline is stabilized by means of a-n interactions with Phe509 and Phe617 3. The cationic head exerts a coulombic interaction with the Aspi" carboxylate and is located in a hydrophobic pocket made of Trp307, Phe616, and Trp613. 4. Finally, the secondary benzylic hydroxyl exchanges a hydrogen bond with Ser410 a FIGURE 2. Interaction capacities of the natural R(-) epinephrine with a model of its receptor Phe617 Trp 307 Ser 410 505 Ser 311 Asp OH Phe 616 HO N Pro 615 - Ser 508 H N Trp 613 Phe509 5 4 3 6 Even taking into account these newer findings, it can be speculated that the Easson Stedman hypothesis still holds. The nonnatural S(+)-adrenaline, having the wrong orientation of its benzylic hydroxyl, is unable to exchange a hydrogen bond with Ser410, and achieves therefore a weaker interaction with the receptor. An alternative model of the adrenergic receptor-active site shows that natural R-(-) epinephrine (adrenaline) can establish a hydrogen bond with the Ser410 alcoholic group, whereas this interaction is not possible with the nonnatural S-(+) epinephrine (Figure 3)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


