Question
Methane gas (CH4) is burning with atmospheric air in a Bunsen burner to produce a stable flame. Determine by assuming negligible dissociation: (a) The
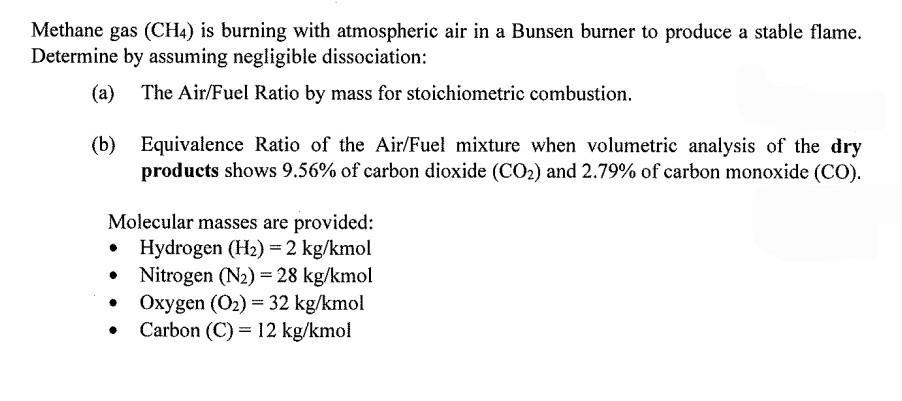
Methane gas (CH4) is burning with atmospheric air in a Bunsen burner to produce a stable flame. Determine by assuming negligible dissociation: (a) The Air/Fuel Ratio by mass for stoichiometric combustion. (b) Equivalence Ratio of the Air/Fuel mixture when volumetric analysis of the dry products shows 9.56% of carbon dioxide (CO) and 2.79% of carbon monoxide (CO). Molecular masses are provided: Hydrogen (H) = 2 kg/kmol Nitrogen (N) = 28 kg/kmol Oxygen (O) = 32 kg/kmol Carbon (C)= 12 kg/kmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Determining AirFuel Ratio and Equivalence Ratio for Methane Combustion To determine the AirFuel Ratio and Equivalence Ratio for the given scenario a A...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Thermodynamics Concepts And Applications
Authors: Stephen R. Turns, Laura L. Pauley
2nd Edition
1107179718, 9781107179714
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



