Question: need help with part C! Learning Goal: To calculate average and relative reaction rates. Consider the reaction Reaction rate can be defined either as the
need help with part C!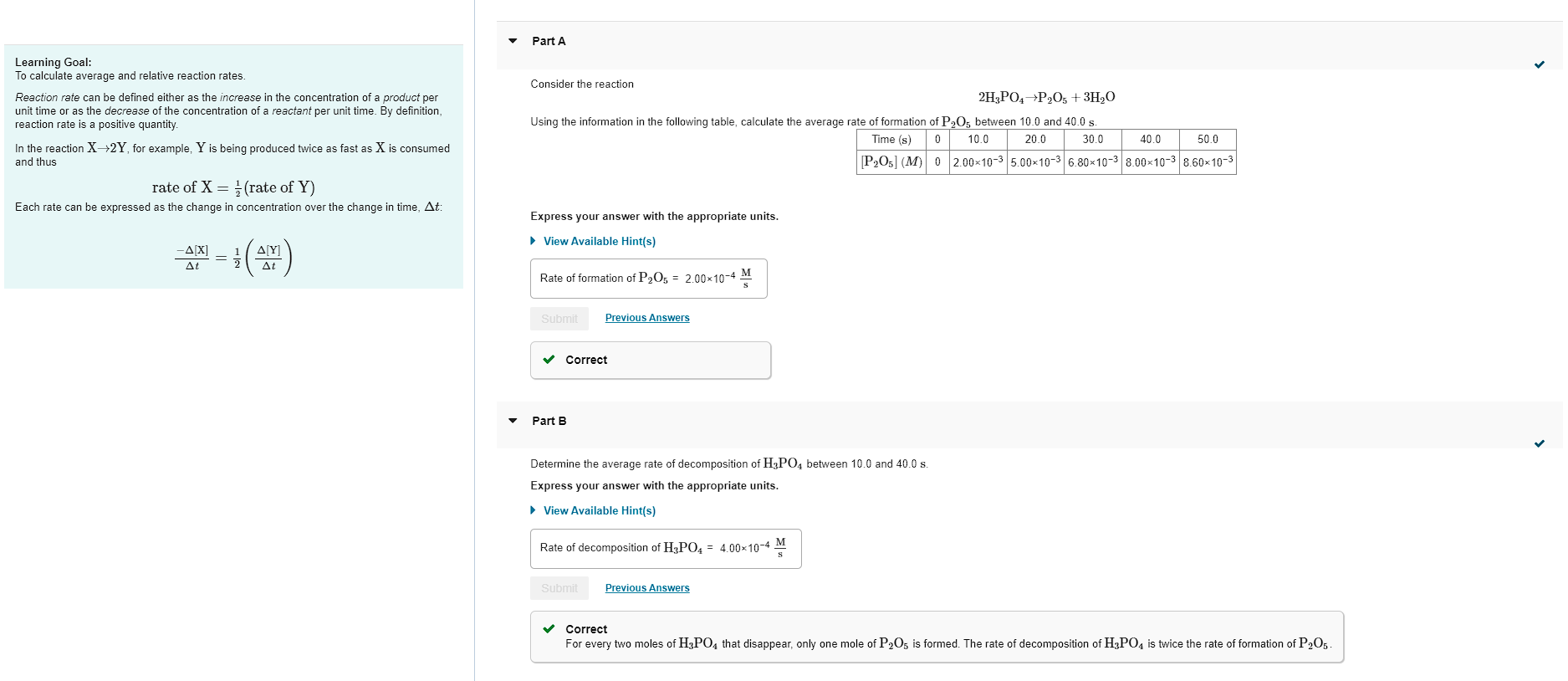
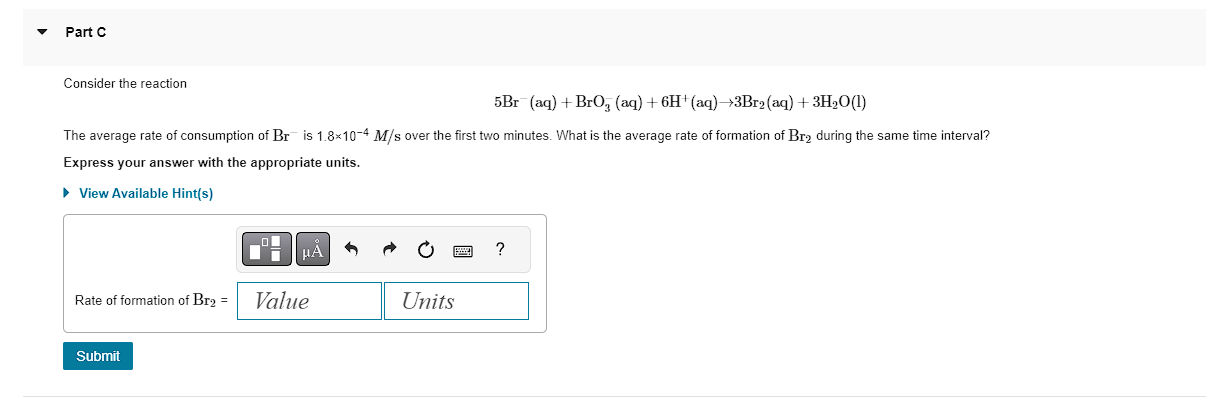
Learning Goal: To calculate average and relative reaction rates. Consider the reaction Reaction rate can be defined either as the increase in the concentration of a product per unit time or as the decrease of the concentration of a reactant per unit time. By definition, reaction rate is a positive quantity. Using the information in the following table, calculate the average ra ,,, In the reaction X2Y, for example, Y is being produced twice as fast as X is consumed and thus rateofX=21(rateofY) Each rate can be expressed as the change in concentration over the change in time, t : Express your answer with the appropriate units. t[X]=21(t[Y]) View Available Hint(s) - Part B Determine the average rate of decomposition of H3PO4 between 10.0 and 40.0s. Express your answer with the appropriate units. View Available Hint(s) Correct For every two moles of H3PO4 that disappear, only one mole of P2O5 is formed. The rate of decomposition of H3PO4 is twice the rate of formation of P2O5. Consider the reaction 5Br(aq)+BrO3(aq)+6H+(aq)3Br2(aq)+3H2O(l) The average rate of consumption of Bris 1.8104M/s over the first two minutes. What is the average rate of formation of Br2 during the same time interval? Express your answer with the appropriate units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


