Question: PLS ANSWER BOTH! WILL UPVOTE FOR BOTH FOR U!! SELECT ALL THAT APPLY!! Which of the following processes has a S>0 ? (Select all that
PLS ANSWER BOTH! WILL UPVOTE FOR BOTH FOR U!!
SELECT ALL THAT APPLY!!
Which of the following processes has a S>0 ? (Select all that apply)
Group of answer choices
Cu(s) at 500 K Cu(s) at 800 K
HCl (aq) + AgNO3 (aq) AgCl (s) + HNO3 (aq)
H2O (l) H2O (s)
CO(g) + NO (g) CO2 (g) + N2(g)
CO2 (s) CO2 (g)
Q2)
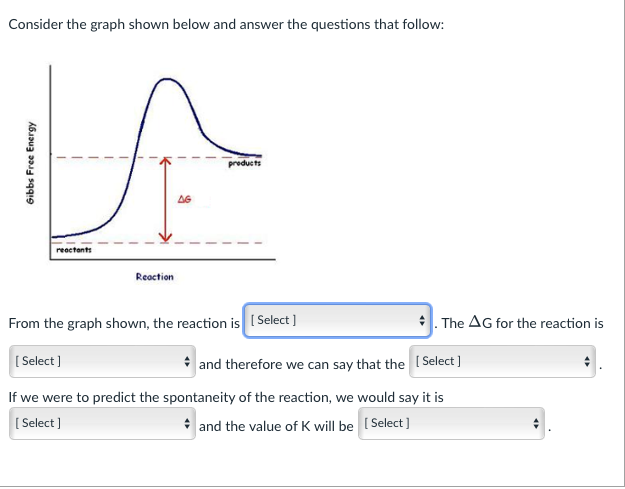
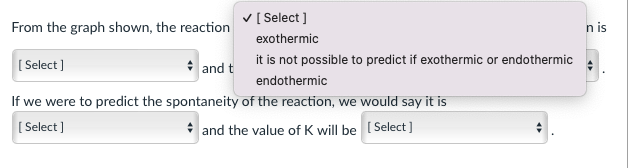
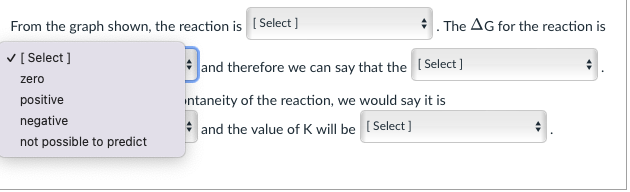
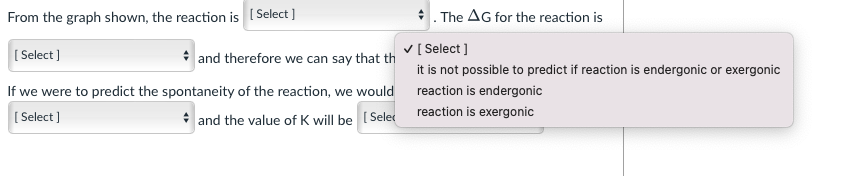
Consider the graph shown below and answer the questions that follow: Gibbs Free Energy A products A6 reactant Reaction From the graph shown, the reaction is (Select] The AG for the reaction is [Select] and therefore we can say that the Select] If we were to predict the spontaneity of the reaction, we would say it is [Select) and the value of K will be (Select] n is [ Select ] From the graph shown, the reaction exothermic Select) and t it is not possible to predict if exothermic or endothermic endothermic If we were to predict the spontaneity of the reaction, we would say it is [Select) and the value of K will be Select] From the graph shown, the reaction is (Select] The AG for the reaction is [Select ] zero positive negative not possible to predict and therefore we can say that the [Select ] Intaneity of the reaction, we would say it is and the value of K will be Select] From the graph shown, the reaction is (Select ] [Select] and therefore we can say that th If we were to predict the spontaneity of the reaction, we would [Select] . and the value of K will be [Selec The AG for the reaction is [Select] it is not possible to predict if reaction is endergonic or exergonic reaction is endergonic reaction is exergonic From the graph shown, the reaction is (Select ] The AG for the reaction is [Select] and therefore we can say that the [Select ] If we were to predict the spontaneity of the reaction, we would say it is [ Select ] spontaneous in the reverse direction ill be (Select] spontaneous in the forward direction impossible to predict the spontaneity of the reaction From the graph shown, the reaction is (Select ] The AG for the reaction is Select] and therefore we can say that the [Select] If we were to predict the spontaneity of the reaction, we would say it is [ Select) and the value of K will b [ Select ] impossible to predict based on the information given. >1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


