Question: Q. 4. Answer the following a. Write a structure for each of the compounds listed. Explain why the name given here is incorrect, and give
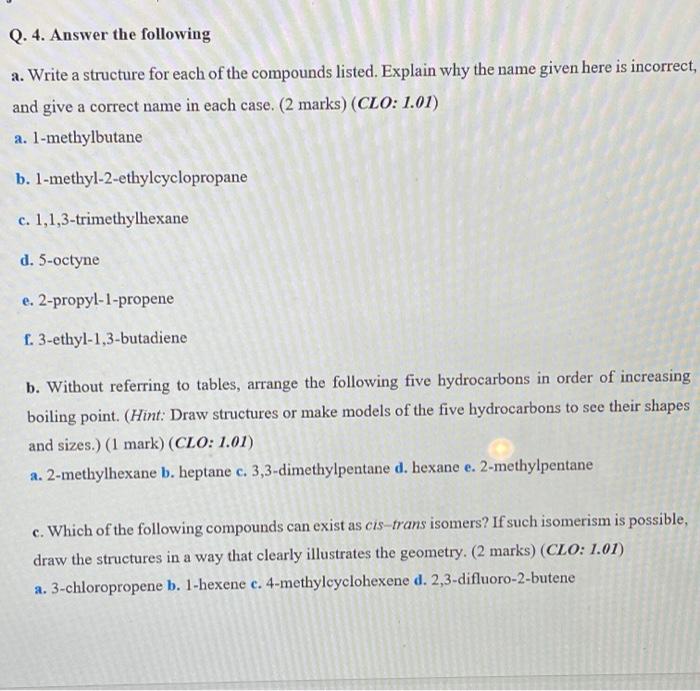
Q. 4. Answer the following a. Write a structure for each of the compounds listed. Explain why the name given here is incorrect, and give a correct name in each case. (2 marks) (CLO: 1.01) a. l-methylbutane b. l-methyl-2-ethylcyclopropane c. 1,1,3-trimethylhexane d. 5-octyne e. 2-propyl-1-propene f. 3-ethyl-1,3-butadiene b. Without referring to tables, arrange the following five hydrocarbons in order of increasing boiling point. (Hint: Draw structures or make models of the five hydrocarbons to see their shapes and sizes.) (1 mark) (CLO: 1.01) a. 2-methylhexane b. heptane c. 3,3-dimethylpentane d. hexane e. 2-methylpentane a c. Which of the following compounds can exist as cis-trans isomers? If such isomerism is possible, draw the structures in a way that clearly illustrates the geometry. (2 marks) (CLO: 1.01) a. 3-chloropropene b. 1-hexene c. 4-methylcyclohexene d. 2,3-difluoro-2-butene a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


