Question: Question 21 4 pts How many grams of P4 (123.88 g/mol) would contain the same number of atoms as 154 g Sg (256.48 g/mol)? 124

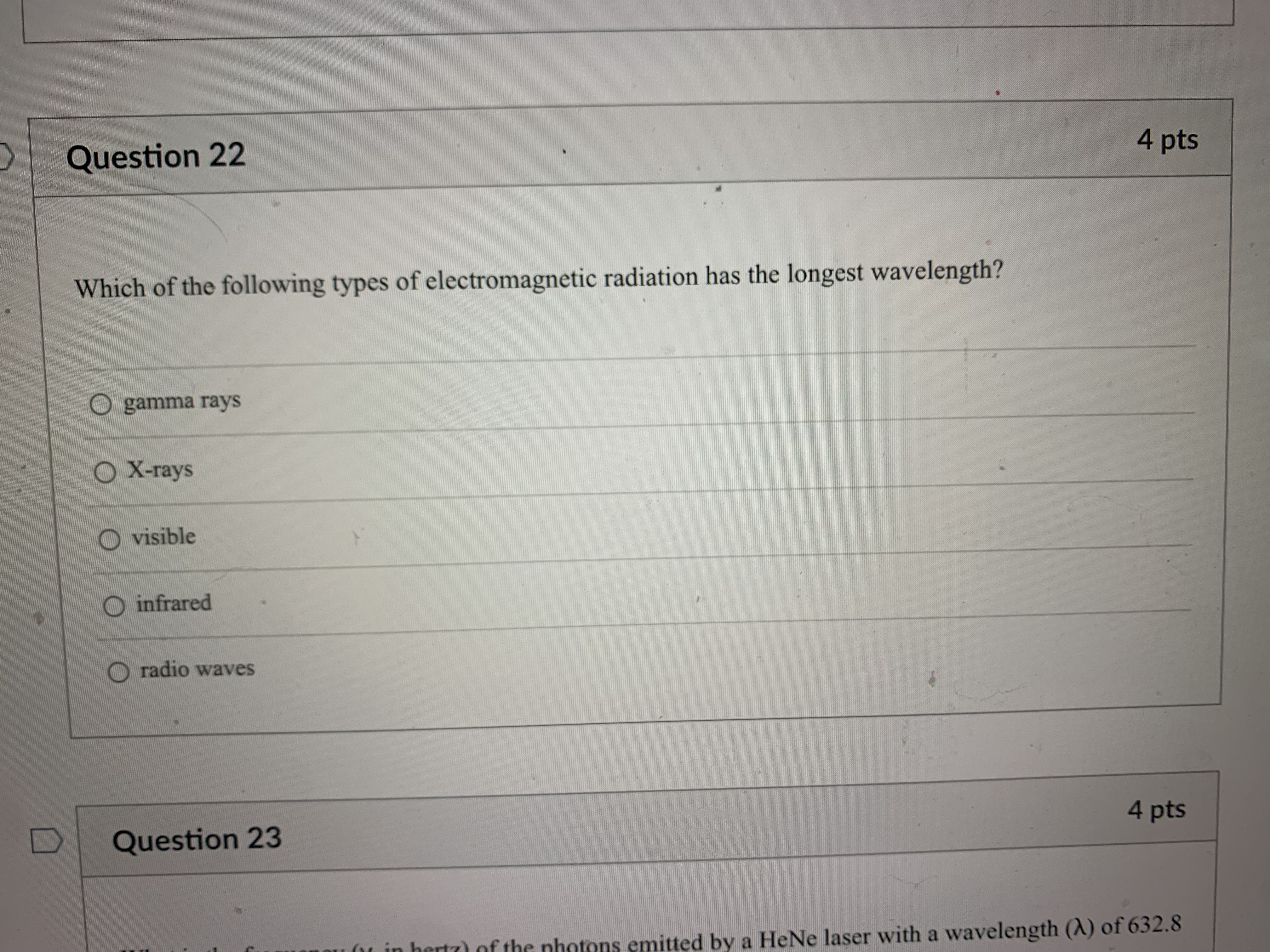
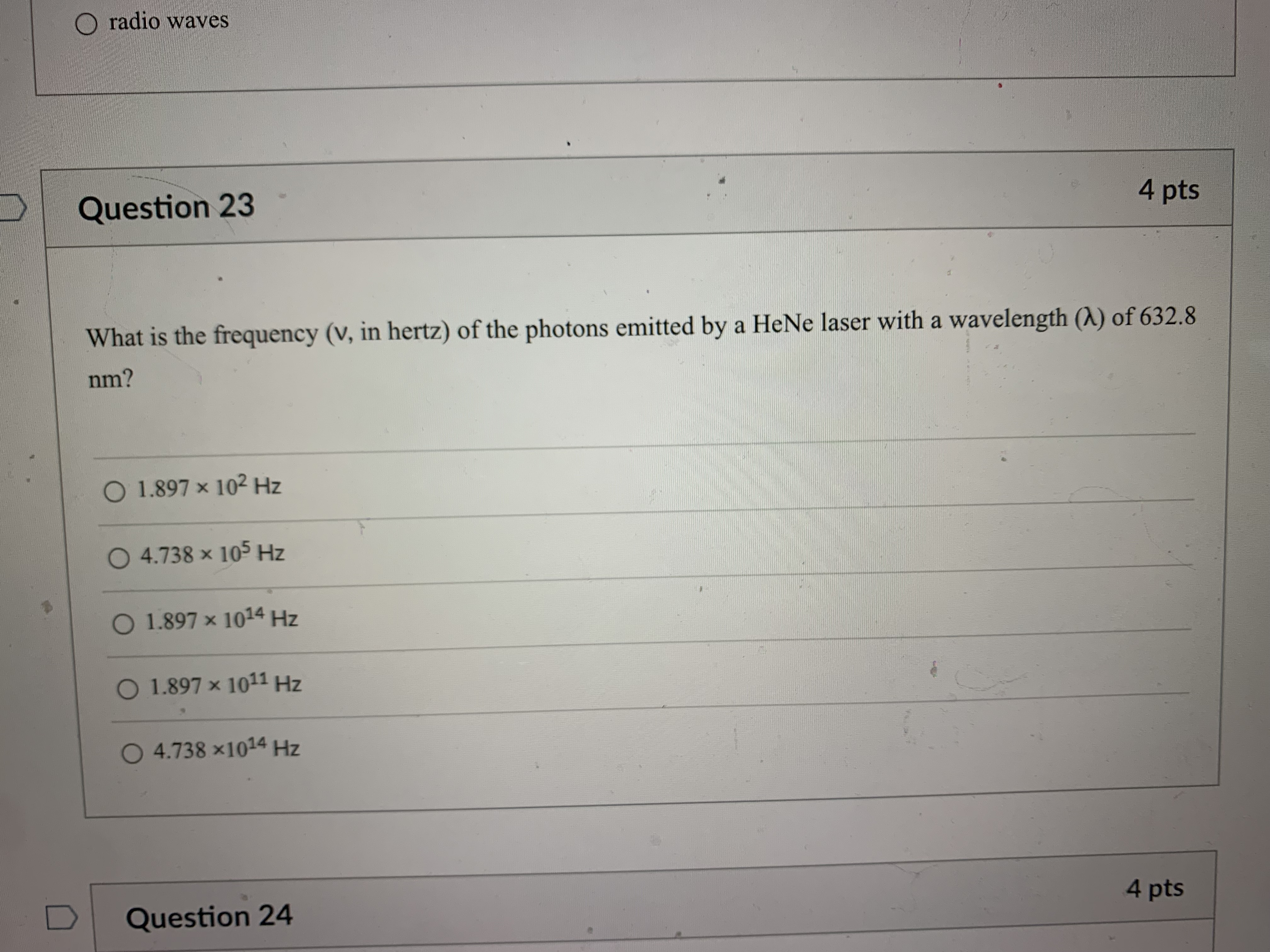
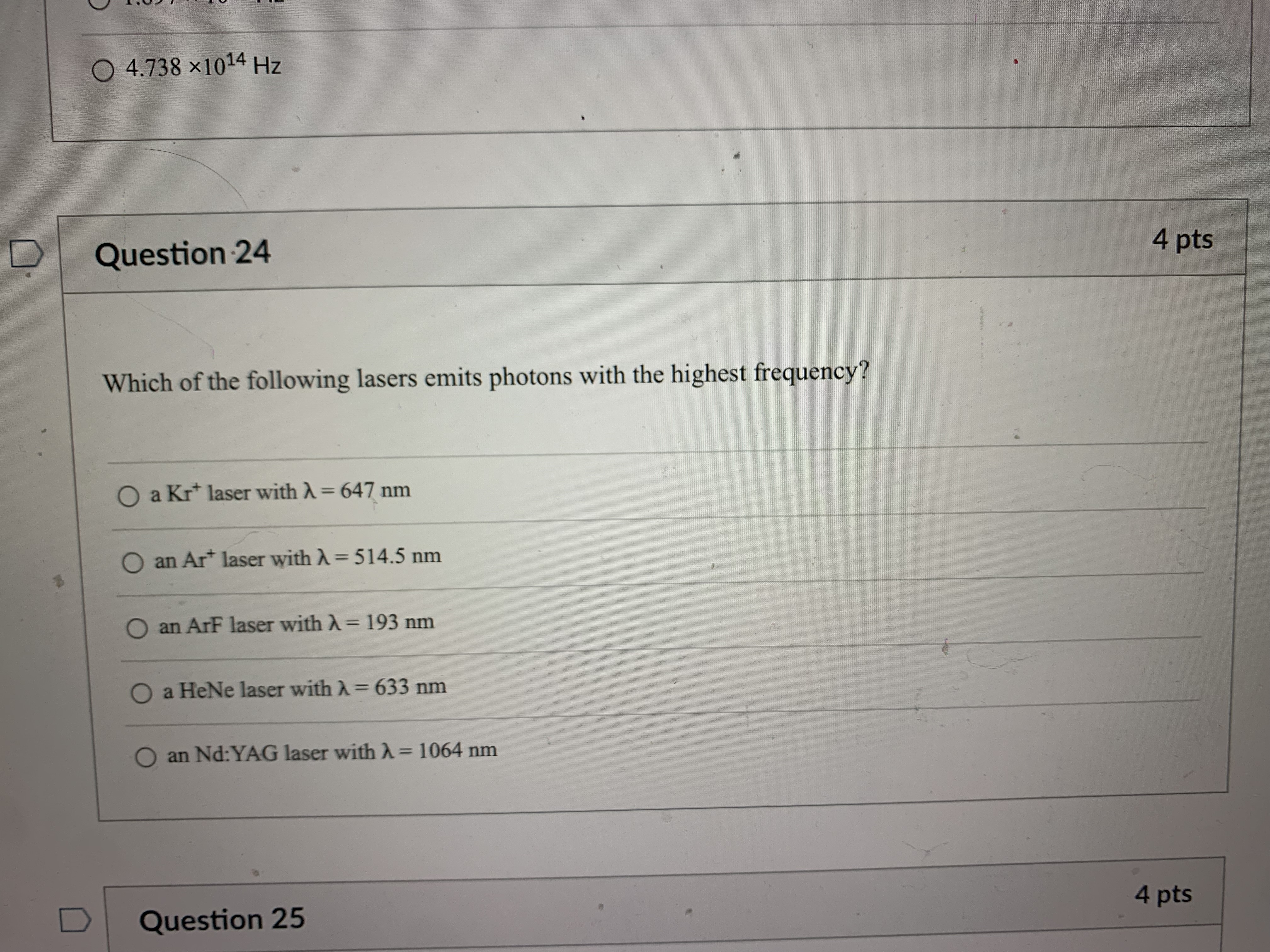
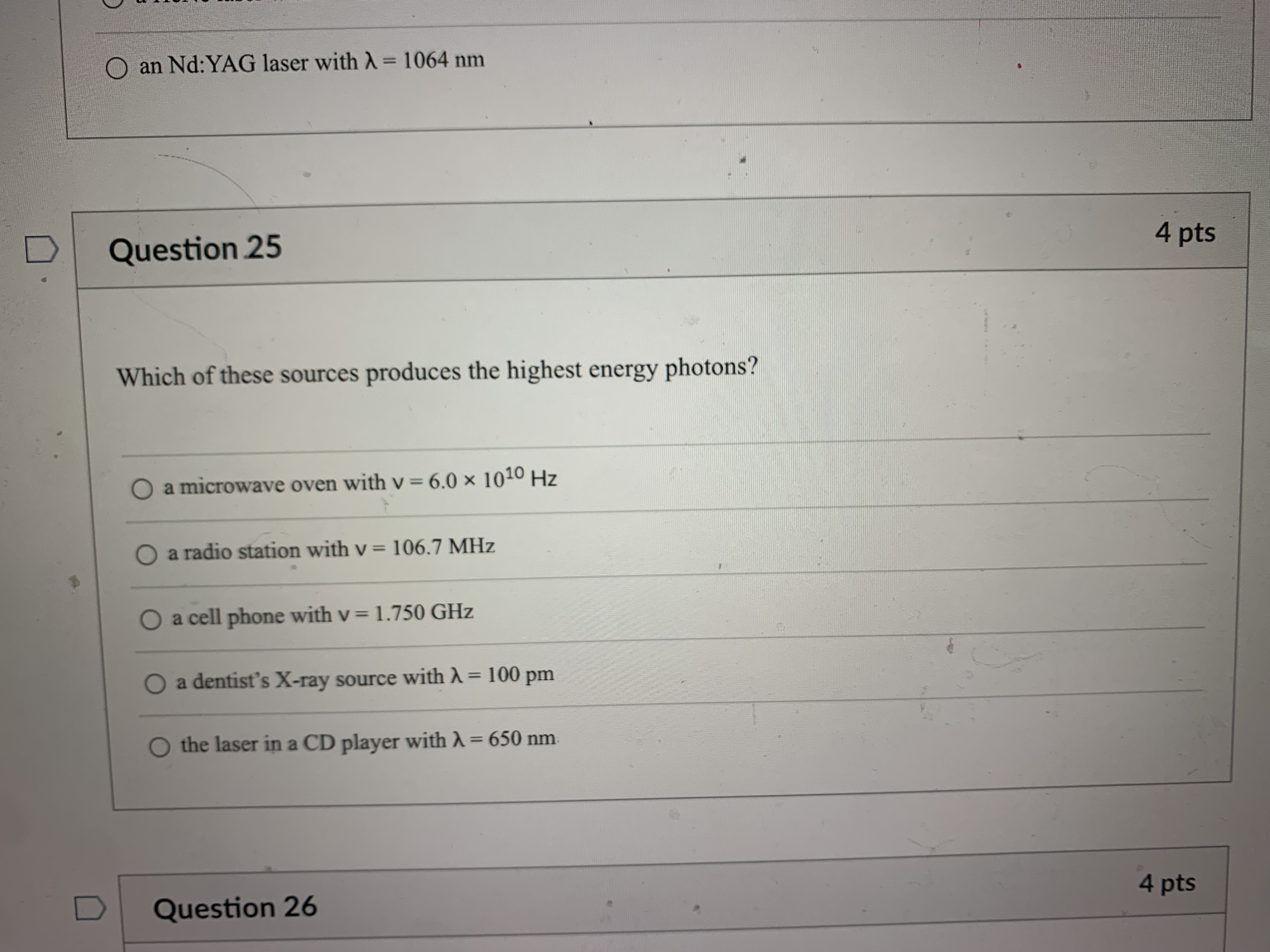

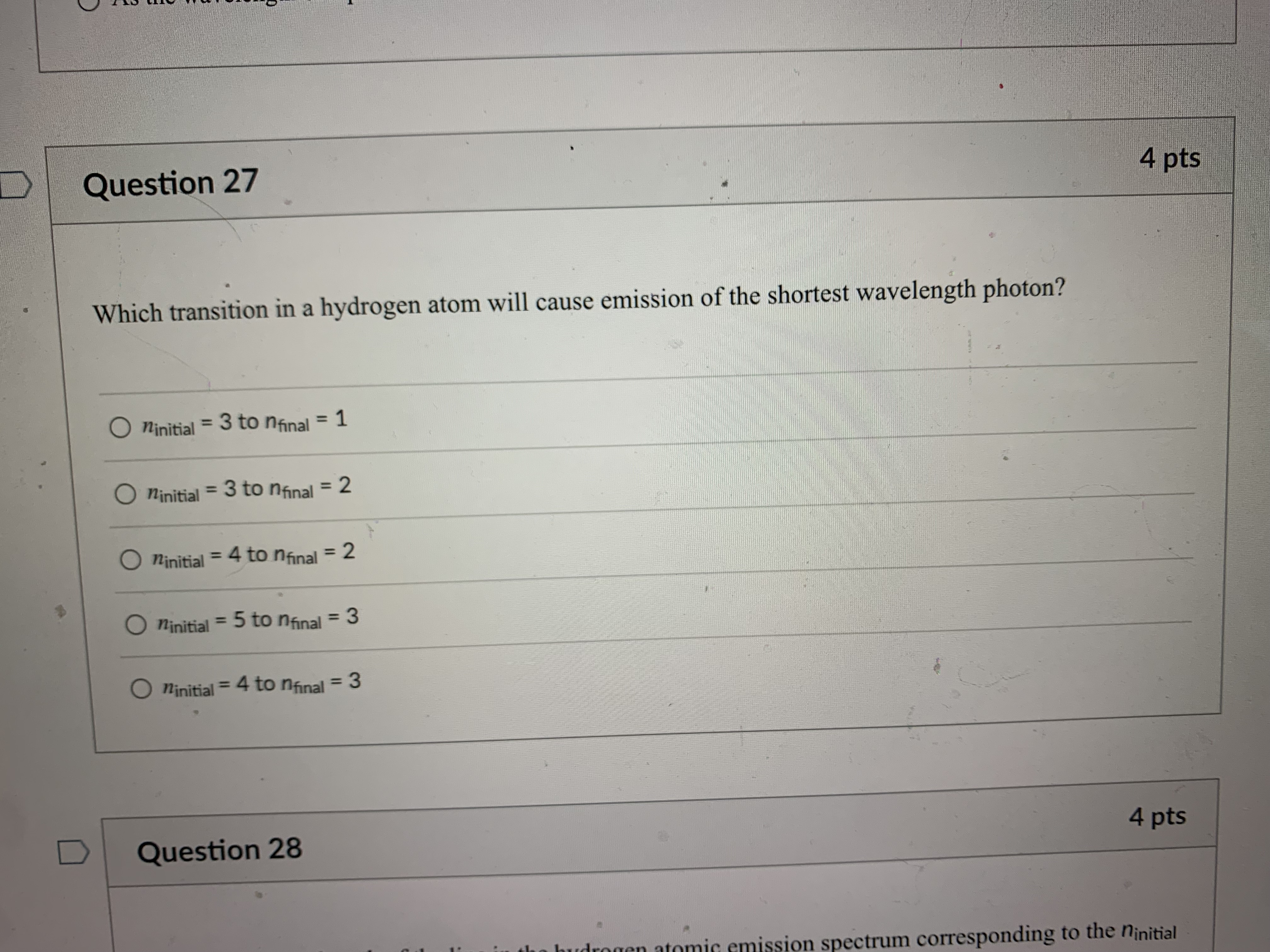
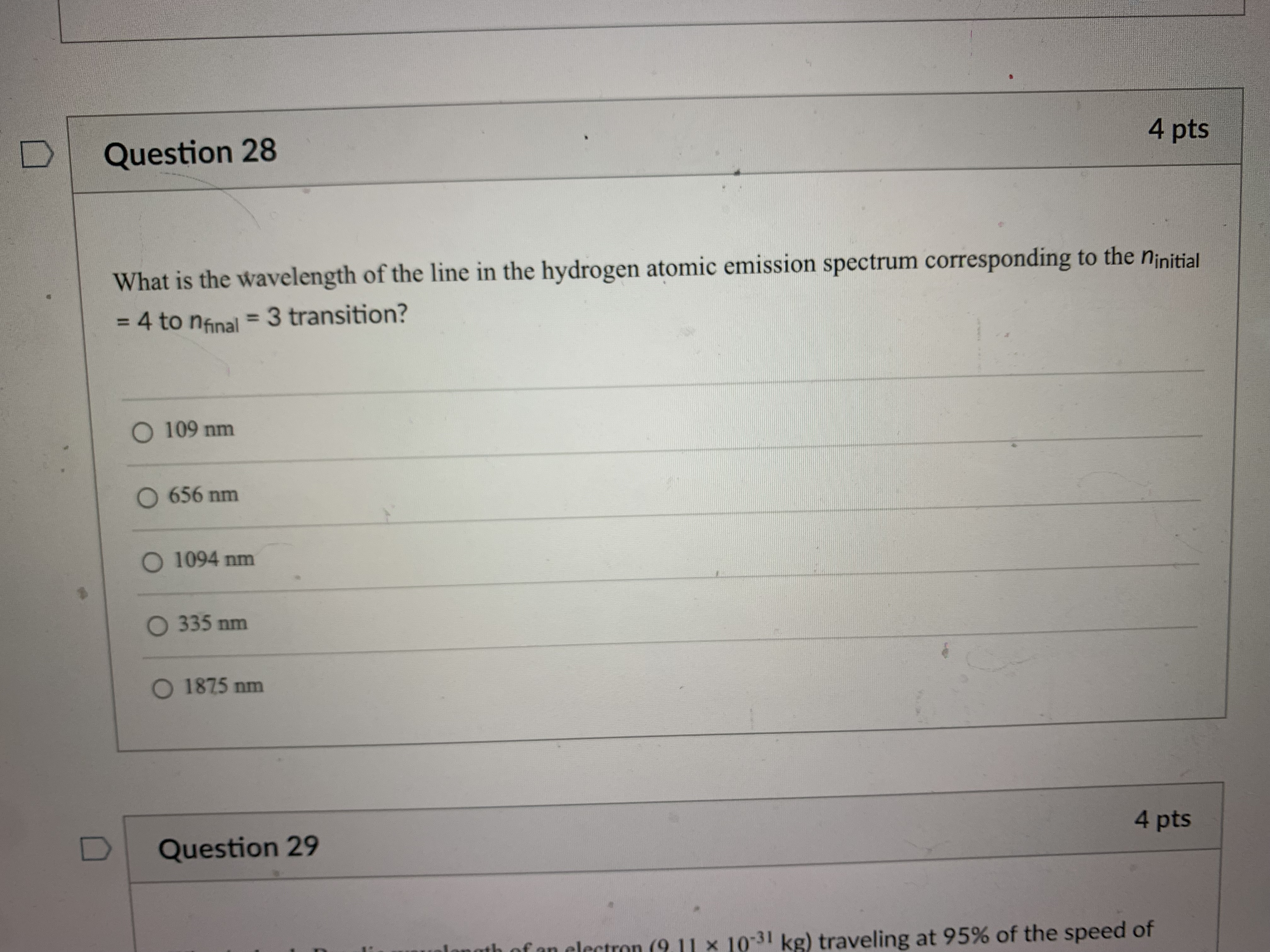

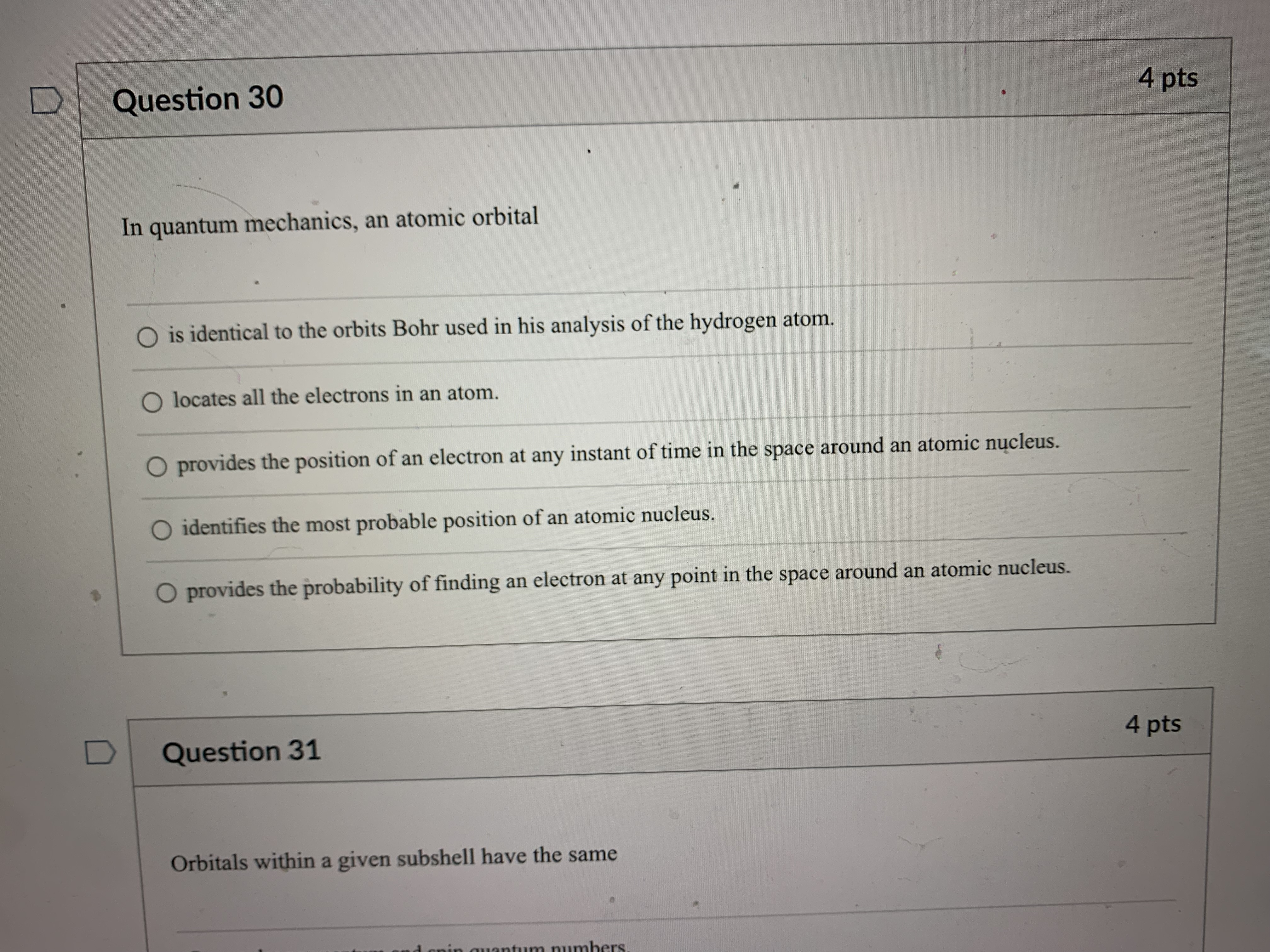
Question 21 4 pts How many grams of P4 (123.88 g/mol) would contain the same number of atoms as 154 g Sg (256.48 g/mol)? 124 g 149 g O 40.2 g O 596 g 18.6 g Question 22 4 ptsQuestion 22 4 pts Which of the following types of electromagnetic radiation has the longest wavelength? gamma rays X-rays visible infrared radio waves Question 23 4 pts emitted by a HeNe laser with a wavelength (A) of 632.8radio waves Question 23 4 pts What is the frequency (V, in hertz) of the photons emitted by a HeNe laser with a wavelength (A) of 632.8 nm? O 1.897 x 102 Hz O 4.738 x 105 Hz O 1.897 x 1014 Hz O 1.897 x 1011 Hz O 4.738 x1014 Hz D Question 24 4 ptsO 4.738 x1014 Hz D Question -24 4 pts Which of the following lasers emits photons with the highest frequency? O a Kr* laser with ) = 647 nm O an Art laser with A = 514.5 nm O an ArF laser with A = 193 nm O a HeNe laser with A = 633 nm O an Nd: YAG laser with A = 1064 nm D Question 25 4 ptsO an Nd: YAG laser with A = 1064 nm D Question 25 4 pts Which of these sources produces the highest energy photons? O a microwave oven with v = 6.0 x 1010 Hz O a radio station with v = 106.7 MHz O a cell phone with v = 1.750 GHz O a dentist's X-ray source with A = 100 pm the laser in a CD player with A = 650 nm D Question 26 4 ptsthe laser in a CD player with A = 650 nm Question 26 4 pts Which of the following statements violates our understanding of photon characteristics? The speed of a photon decreases as its wavelength decreases. O Light can be viewed as packets of energy. As the frequency of a photon decreases, its energy also decreases. O A high-energy photon has a shorter wavelength than a low-energy photon. O As the wavelength of a photon decreases, its energy increases. D Question 27 4 ptsQuestion 27 4 pts Which transition in a hydrogen atom will cause emission of the shortest wavelength photon? O ninitial = 3 to nfinal = 1 O ninitial = 3 to nfinal = 2 O ninitial = 4 to nfinal = 2 O ninitial = 5 to nfinal = 3 O ninitial = 4 to nfinal = 3 D Question 28 4 pts sion spectrum corresponding to the ninitial\fQuestion 29 4 pts What is the de Broglie wavelength of an electron (9.11 x 10-31 kg) traveling at 95% of the speed of light? O 240 pm O 2.6 fm O 2.6 pm O 2.4 pm O 24 fm D Question 30 4 ptsQuestion 30 4 pts In quantum mechanics, an atomic orbital O is identical to the orbits Bohr used in his analysis of the hydrogen atom. O locates all the electrons in an atom. O provides the position of an electron at any instant of time in the space around an atomic nucleus. O identifies the most probable position of an atomic nucleus. O provides the probability of finding an electron at any point in the space around an atomic nucleus. D Question 31 4 pts Orbitals within a given subshell have the same
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


