Question: Suggest Question 1. Consider the solvolysis of the two isomeric chiral, optically active p-nitrobenzoates 10 and 11. (a) Draw the structure of the free ion
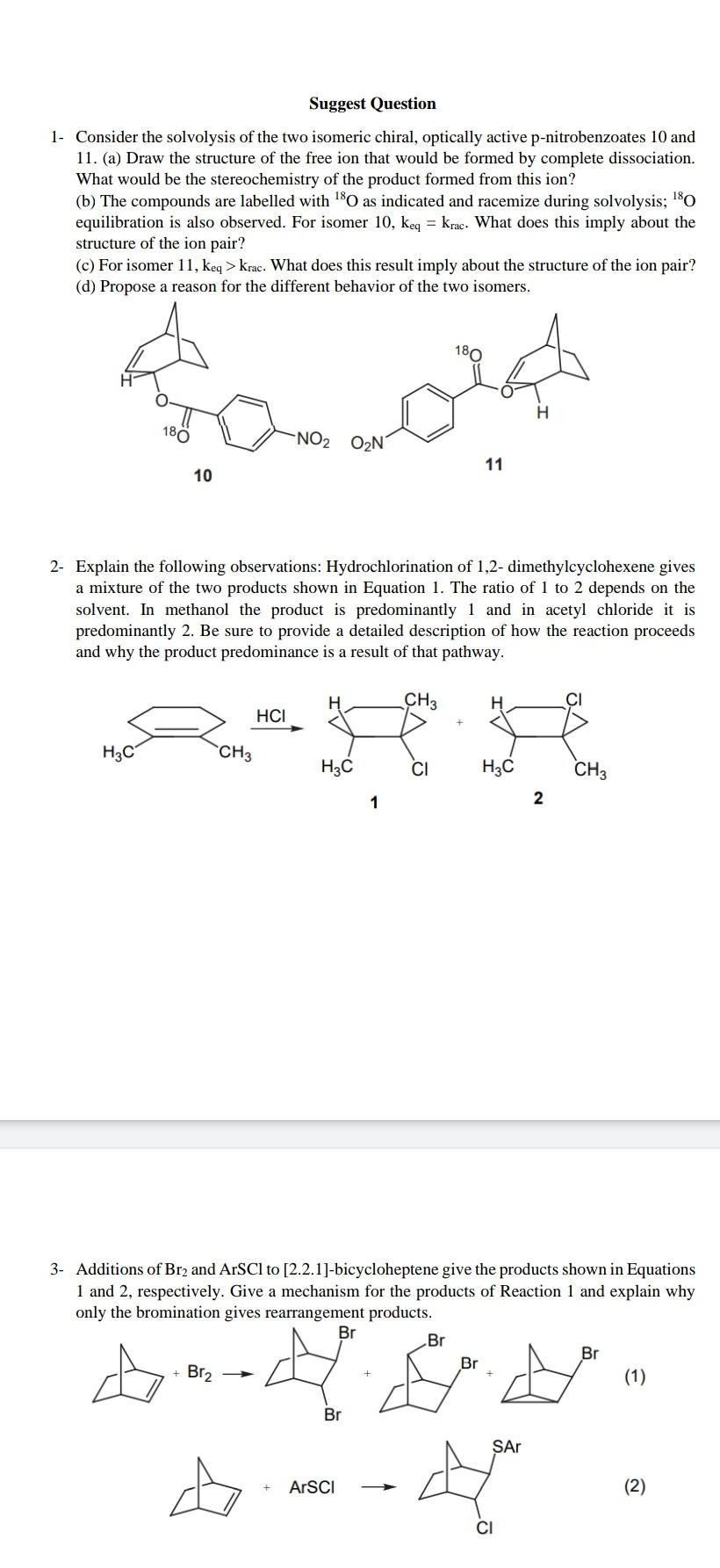
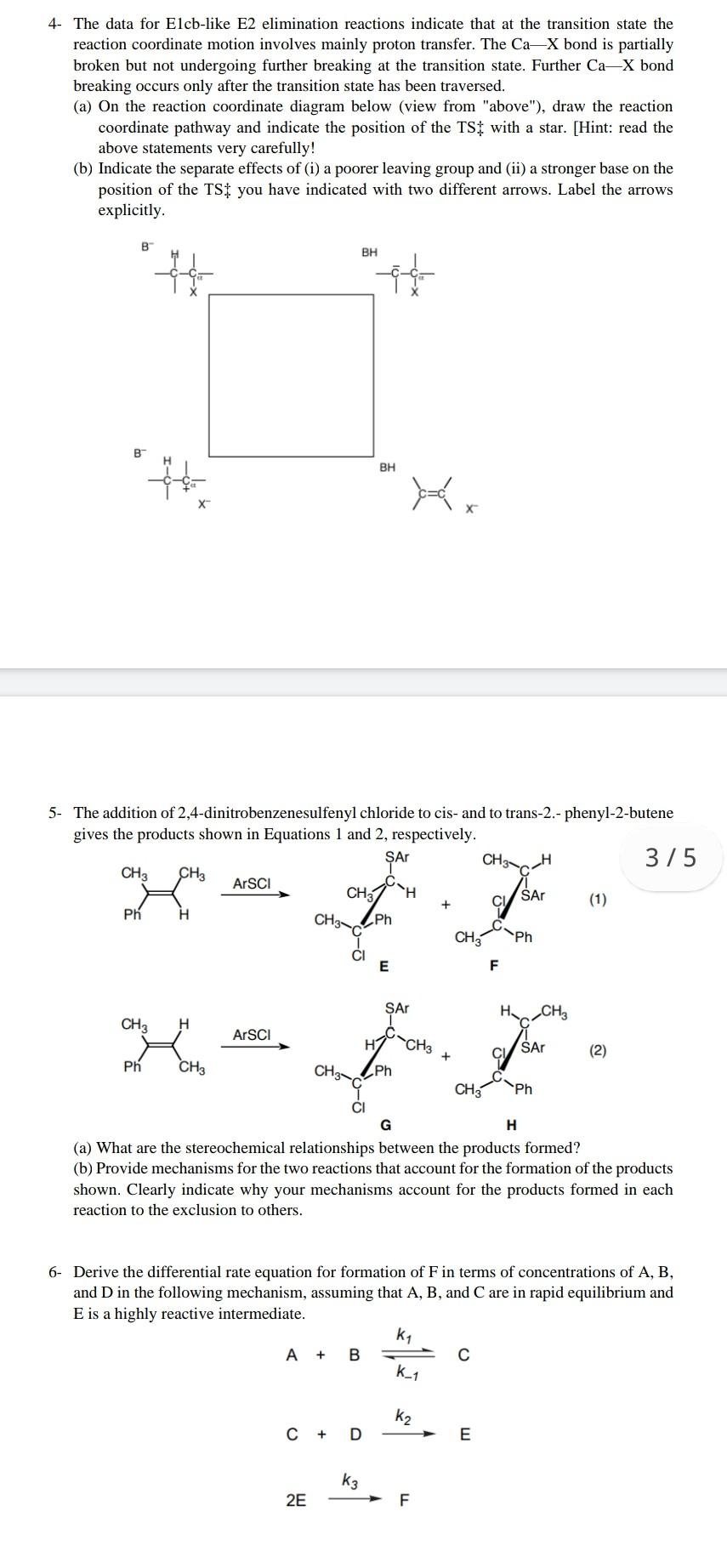
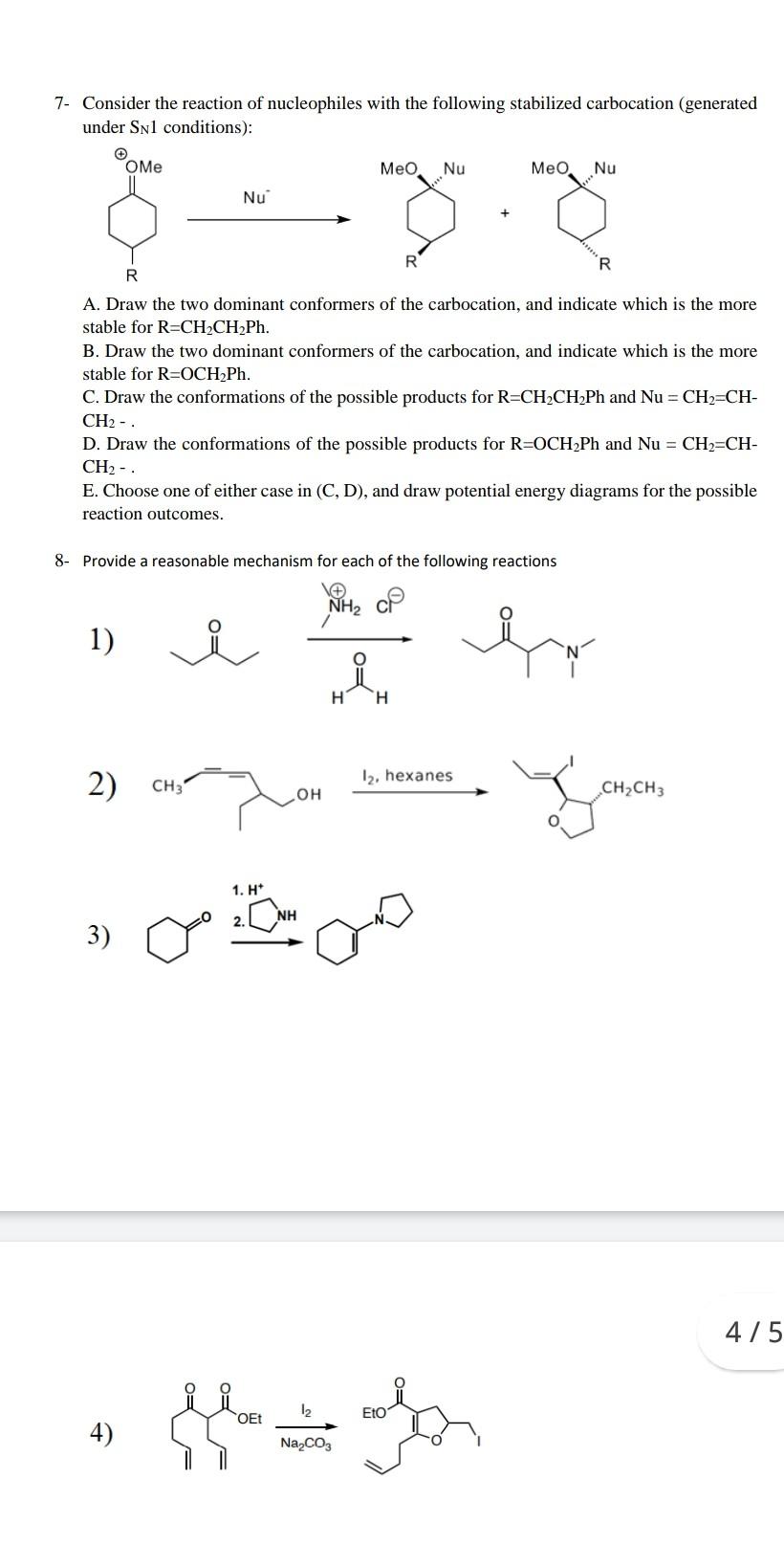
Suggest Question 1. Consider the solvolysis of the two isomeric chiral, optically active p-nitrobenzoates 10 and 11. (a) Draw the structure of the free ion that would be formed by complete dissociation. What would be the stereochemistry of the product formed from this ion? (b) The compounds are labelled with 180 as indicated and racemize during solvolysis; 180 equilibration is also observed. For isomer 10, keq = krac. What does this imply about the structure of the ion pair? (c) For isomer 11, keq > krac. What does this result imply about the structure of the ion pair? (d) Propose a reason for the different behavior of the two isomers. 180 180 NO2 ON 11 10 2- Explain the following observations: Hydrochlorination of 1,2-dimethylcyclohexene gives a mixture of the two products shown in Equation 1. The ratio of 1 to 2 depends on the solvent. In methanol the product is predominantly 1 and in acetyl chloride it is predominantly 2. Be sure to provide a detailed description of how the reaction proceeds and why the product predominance is a result of that pathway. a H CH3 HCI H3c CH3 H2C CI H2C CH3 1 N 3- Additions of Br2 and ArSCI to [2.2.1]-bicycloheptene give the products shown in Equations 1 and 2, respectively. Give a mechanism for the products of Reaction 1 and explain why only the bromination gives rearrangement products. Br Br Br Br + Br2 (1) Br SAT ArSCI (2) CI 4- The data for Elcb-like E2 elimination reactions indicate that at the transition state the reaction coordinate motion involves mainly proton transfer. The CaX bond is partially broken but not undergoing further breaking at the transition state. Further Ca-X bond breaking occurs only after the transition state has been traversed. (a) On the reaction coordinate diagram below (view from "above"), draw the reaction coordinate pathway and indicate the position of the TSI with a star. (Hint: read the above statements very carefully! (b) Indicate the separate effects of (i) a poorer leaving group and (ii) a stronger base on the position of the TSI you have indicated with two different arrows. Label the arrows explicitly. B BH * " BH 5. The addition of 2,4-dinitrobenzenesulfenyl chloride to cis- and to trans-2.-phenyl-2-butene gives the products shown in Equations 1 and 2, respectively. SAT CH 375 CH3 CH3 ArSCI CH H SAT (1) Ph CH/Ph Ph CH3 E F SAT H CH ArSCI HY CH CHAN/Ph CHE /Sar (2) + Ph CH, CH3 Ph G H (a) What are the stereochemical relationships between the products formed? (b) Provide mechanisms for the two reactions that account for the formation of the products shown. Clearly indicate why your mechanisms account for the products formed in each reaction to the exclusion to others. 6- Derive the differential rate equation for formation of F in terms of concentrations of A, B, and D in the following mechanism, assuming that A, B, and C are in rapid equilibrium and E is a highly reactive intermediate. k1 A + B k_1 k2 C + D E ka F 2E 7- Consider the reaction of nucleophiles with the following stabilized carbocation (generated under Snl conditions): Me Nu Med Nu Nu 8. R R A. Draw the two dominant conformers of the carbocation, and indicate which is the more stable for R=CH2CH Ph. B. Draw the two dominant conformers of the carbocation, and indicate which is the more stable for R=OCH Ph. C. Draw the conformations of the possible products for R=CH2CH2Ph and Nu = CH2=CH- CH2 - D. Draw the conformations of the possible products for R=OCH Ph and Nu = CH2=CH- CH2 - E. Choose one of either case in (C, D), and draw potential energy diagrams for the possible reaction outcomes. 8- Provide a reasonable mechanism for each of the following reactions NH2 1) H H 2) 12. hexanes CH31 OH CH2CH3 1. H* 2. 3) 4/5 OEt 12 Eto 4) Hair Na2CO3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


