Question: the only gas present stone time it important to note that the reactions take place over water or in Bueous medium. The reaction vessel therefore

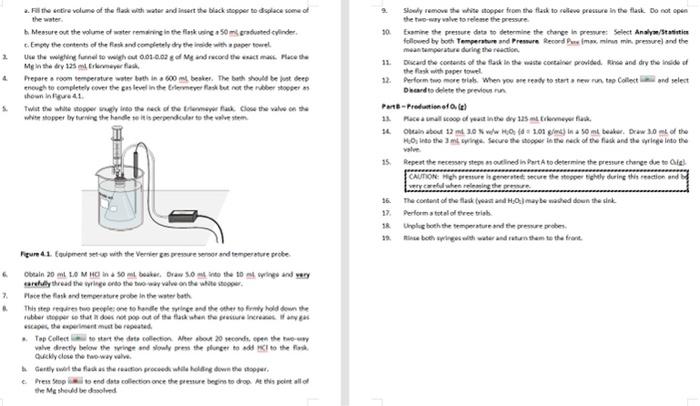
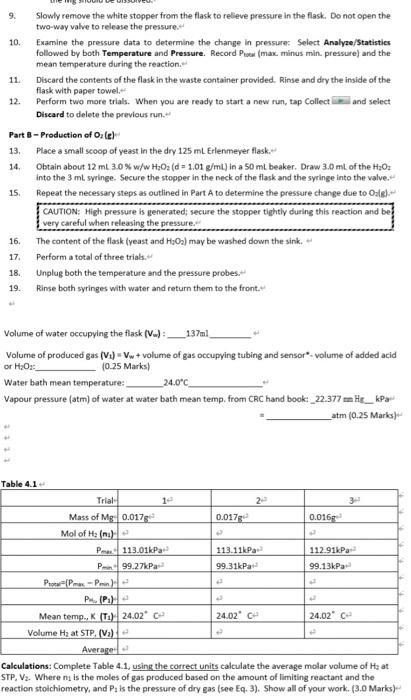
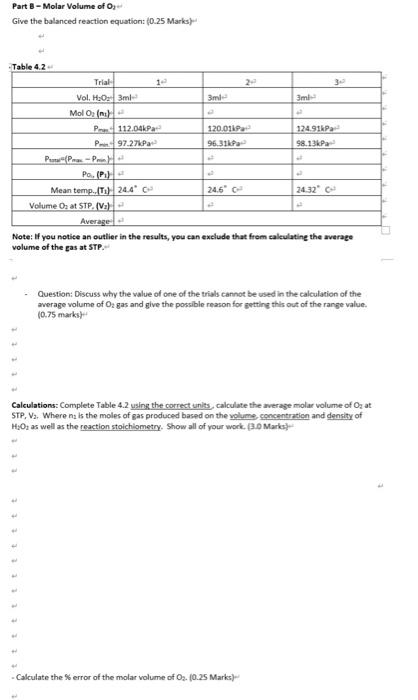


the only gas present stone time it important to note that the reactions take place over water or in Bueous medium. The reaction vessel therefore also contains pressure contributed by water vapour. Toure a proper reading of get practure for the dy gas component, the partial roro due to the water vapour component must be counted for according Dalton's Law Pau - Puse Poi 33 Another application of the ideal gat daw is to measure the density of a gas if we know the molar volume of an ideal gas at ST we can determine the density of the under these conditions The density of at STP is given by the following relationship solar mas mola velme 14 Notice that deroity is directly propertimalto moler mess. We would therefore expect an increase in density with increasing moler mass when comparing the standard dereity of Trogen and oxygen BAL Additional Review Materi Relevant sections in the test Chemistry for Engineering Students, 4 Ed, Brown, olmek 53-55 Background Some of the earliest and most sgrifiant quantitate experiments reveal the four variables are ully suficient to define the state or condition of a gas temperature (1): pressure i volume (V and the quantity of matter, usually expressed as the number of molested. These four variables are Interdependent, L., any one of them can be determined by measuring the other three. The key relationships between the variables se Boyle's Charles's, and Avogadres de los Cach of these teve expresses the electione volle on mother when the remaining wewe held constant. A convenient combination of these simple gas law is the ideal gas law which describes the relationship twin the proposes P. V. Land for any gasbehaving as an ideal gas Equition 11 P-ART Where is the wiweral gas contact 1 = 0.082068 animo") and has the same value for all cases The Ideel twise when applied to see set of condition. It is often required wer, to compare two parte condition is use the ideal gas equation can be applied to both the initial and final conditions the ideal gasestion can then be rewritten as a generales gation Custion : V. 03 One slation of the soul www to we the molar volume of a ps Molar volume is defines the volume cupied by one more of a STP Arendo prostulated that the same temperature and pressure ou volumes of gettin the same number of molecules This statement is nown as Avogadrok law. Thus, the number of roles of molecules of a gas na samle of a given volume, ata ven omgerators and grow does not depend on the det the intellit wedermine the volume of a new quantity of Ostrow temperature and the ple to determine the women mot Tsmo wil at STP, sed by Magdoll law, thout be the same for alle her we will come molar volume of both handen separately in order to writy Avogadro's wonded to the rain mesum metal with of grous hydrocork wed whereason he produced through the decomposition reaction of hydrogen peroxide in noves solution Although the decomposition of too, and Hosporaneous rocell, cur dowly. We can do the reaction record by the addition of a cataly called synes is all The prese change between the land inalconditioned to caluate the world by one mole of standard operate 22.15 Kand preven. For an ideal gas this volumes 22.414 LatSTF Dato's law of Parcial Pressures vates that the rest of mature sels the sun the partial pressure of the componenter Although it may seem that either hydrogen er over Gware & quipment Bree 100011 Two hole white rubber stopper Bakr 600m Vernier gepresenter Hack rubber stopper Versier Latest Interface termeer as 123 Vorrer temperature robe Graduated cylinder 50 m Weighinguel Syringe om, 10 ml. valve and thing Chemicals & Regent LOM drecho CC 10% w/w Hideal Maresme Melal Active dry yet CAUTIONE ICE Bicol with an Procedure Part-Praditionelle 1 Power on the table power button), The Last op dhe automately Connect tenperature robe to the port and pressure were to CH 2. The Gert op will to the connected to delivereadre will opeor in the Meter ren. The wide Tindad Mod with a whole mwend warf the pressure with is change it tot by clicking on the premureve reading and from the rapomeru select Change united then 2 Determine the valla volume ina 125 termeyerland which the cases will 2 File satire volume of the flade with water and insert the black stopper to displace some of the water Messure out the volume of water remaining in the laskine 50m raduted cylinder Engey the contents of the fiestand completely dy there with per towel Uite the fun to what 0.01-0.02 Mg and record the mass Muce the Mg in theory 125 Bremerfis Prepare a room temperature water both in at besker. The bath should be deep enough to completely over the pas level in the termeverlast but not the be opper shown 41 Twith while stopper way the neck of tree as close the value on se white stopper by turning the handle siti perpendicular to the valvester 2 Stoly remove the topper for the flad to see pescure in the lack Do not open the two-way valve to release the pressure 10 Examine the pressure data to determine the change in pressure Select Analy/Statisti followed by both Temperature and Pressure Record Primam in pressure and the mean tamperture during the reaction 11. Discard the contents of the face in the weste container provided in and dry the wide of the flask with paper towel 12. Perform two more trial. When you we ready to start a new on the Collect and select Dhard to delete the previous Partielle 13. Rocca al coop of you in the dy 125 mlrlenmeyer fuck 14. Olain about 12 mt 20 H 3.01 ) Insomt beter. Drew 30 of the HO Into the ringe Sexure the shop the neck of the fuck and the wing Into the TE 15 Repeat the necessary step outlined in Port to determine the pressure change de total CAUTION Moh premre ha generated secure the stepper tightly during the reaction and 16. The content of the flask wat and maybe wached down the link 17. Perform total of three trial * Urolig bh the temperature and the pressure robes bo wings with water and tutun shum to the front. Figura 41 Covprent set with the Versier preure root and temperature probe Obtain 20 LO MC 50 ob Draw 3.0 to the 10 and we carly thread the wingerds the way value on the white show Place them and temperature probe in the water both This step remareste people one to handle the winged the other of hold down the rubber per te that does not pop out of the face when the return capas, the partma to reportis, Top Collect to start the data collection Altersbout 20 seconds, open the mouw valve directly below the wing and slowly pre the plunger to add to the most Quickly close the two-way valve Garty wet the fasthern proceed while down the oper Presepto end data collection once the pressure begin to drop At this point all of de Method 9. 10. Slowly remove the white stopper from the flask to relieve pressure in the flask. Do not open the two-way valve to release the pressure Examine the pressure data to determine the change in pressure Select Analyze/Statisties followed by both Temperature and Pressure. Record Prot (max. minus min. pressure) and the mean temperature during the reaction. Discard the contents of the flask in the waste container provided. Rinse and dry the inside of the flask with paper towel. 12. Perform two more trials. When you are ready to start a new run, tap Collect and select Discard to delete the previous run. Part 8 - Production of Orle 13. Place a small scoop of yeast in the dry 125 mL Erlenmeyer flask. 14. Obtain about 12 mt 3.0 % w/w H:02 (d = 1.01 g/ml) in a 50 ml beaker. Draw 3.0 ml of the H20; into the 3 ml syringe. Secure the stopper in the neck of the flask and the syringe into the valve. 15. Repeat the necessary steps as outlined in Part A to determine the pressure change due to Oale CAUTION: High pressure is generated; secure the stopper tightly during this reaction and be very careful when releasing the pressure The content of the flask (yeast and Hu0) may be washed down the sink. Perform a total of three trials. 18. Unplug both the temperature and the pressure probes. 19. Rinse both syringes with water and return them to the front. 16. 17. Volume of water occupying the flask (v.):__137m1_ Volume of produced gas (V+) =Vw + volume of gas occupying tubing and sensor volume of added acid or H2O2: (0.25 Marks) Water bath mean temperature: _24.0C Vapour pressure (atm) of water at water bath mean temp. from CRC hand book_22.377 He_kPa _atm (0.25 Marks) tttt Table 4.1 Trial Mass of Mg 0.017 0.017 0.0169 Mol of Hamile P113.01kPa 113.11kPa 112.91kPa Pon99.27kPa 99.31kPa 99.13kPa PP-P) P.PH 3 Mean temp., K (T) 24.02 C 24.02 C 24.02 C Volume Hz at STP. (Va) Average: Calculations: Complete Table 4.1, using the correct units calculate the average molar volume of Heat STP. V. Where ns is the moles of gas produced based on the amount of limiting reactant and the reaction stoichiometry, and P, is the pressure of dry gas (see Eq. 3). Show all of your work. (3.0 Marks) Part 8 - Molar Volume of O, Give the balanced reaction equation: (0.25 Marks Table 4.2 Trial Vol. HO 3ml 3ml 3m Mol Ol P112,04P 120.01 124.91P P97.27 Pa 96.31P 98.13 Pa PPP Po.PH Mean temp. (T) 24.4 24.6" - 24.32 Volume 03 at STP. (2) Average Note: If you notice an outlier in the results, you can exclude that from calculating the werage volume of the gas at STP. Question: Discuss why the value of one of the trials cannot be used in the calculation of the average volume of Oz gas and give the possible reason for getting this out of the range value. (0.75 marks Calculations: Complete Table 4.2 using the correct units calculate the average molar volume of Oz at STP, V. Where na is the moles of gas produced based on the volume concentration and density of H:Os as well as the reaction stoichiometry. Show all of your work 3.0 Marks Calculate the error of the molar volume of Oz. (0.25 Marks) - Calculate the density (in g/L) of both hydrogen and oxygen gas from average molar volumes at STP. (1.0 Marks) Hypothesis [2.0] A hypothesis summarizes what outcomes you anticipate for the experimental procedure. Typically the outcomes will be presented in terms of the relationship between dependent and independent variables. Give the reason for your hypothesis based on what you know about the scientific concept of the lab and how that knowledge led you to the hypothesis. Discussion (3.0) Explain how the data supports, or does not support, your hypothesis. Discuss the accuracy (% error) between experimental and expected values in regards to molar volume and density. Identify at least one source of error. How does this error affect the data and how could it be improved? the only gas present stone time it important to note that the reactions take place over water or in Bueous medium. The reaction vessel therefore also contains pressure contributed by water vapour. Toure a proper reading of get practure for the dy gas component, the partial roro due to the water vapour component must be counted for according Dalton's Law Pau - Puse Poi 33 Another application of the ideal gat daw is to measure the density of a gas if we know the molar volume of an ideal gas at ST we can determine the density of the under these conditions The density of at STP is given by the following relationship solar mas mola velme 14 Notice that deroity is directly propertimalto moler mess. We would therefore expect an increase in density with increasing moler mass when comparing the standard dereity of Trogen and oxygen BAL Additional Review Materi Relevant sections in the test Chemistry for Engineering Students, 4 Ed, Brown, olmek 53-55 Background Some of the earliest and most sgrifiant quantitate experiments reveal the four variables are ully suficient to define the state or condition of a gas temperature (1): pressure i volume (V and the quantity of matter, usually expressed as the number of molested. These four variables are Interdependent, L., any one of them can be determined by measuring the other three. The key relationships between the variables se Boyle's Charles's, and Avogadres de los Cach of these teve expresses the electione volle on mother when the remaining wewe held constant. A convenient combination of these simple gas law is the ideal gas law which describes the relationship twin the proposes P. V. Land for any gasbehaving as an ideal gas Equition 11 P-ART Where is the wiweral gas contact 1 = 0.082068 animo") and has the same value for all cases The Ideel twise when applied to see set of condition. It is often required wer, to compare two parte condition is use the ideal gas equation can be applied to both the initial and final conditions the ideal gasestion can then be rewritten as a generales gation Custion : V. 03 One slation of the soul www to we the molar volume of a ps Molar volume is defines the volume cupied by one more of a STP Arendo prostulated that the same temperature and pressure ou volumes of gettin the same number of molecules This statement is nown as Avogadrok law. Thus, the number of roles of molecules of a gas na samle of a given volume, ata ven omgerators and grow does not depend on the det the intellit wedermine the volume of a new quantity of Ostrow temperature and the ple to determine the women mot Tsmo wil at STP, sed by Magdoll law, thout be the same for alle her we will come molar volume of both handen separately in order to writy Avogadro's wonded to the rain mesum metal with of grous hydrocork wed whereason he produced through the decomposition reaction of hydrogen peroxide in noves solution Although the decomposition of too, and Hosporaneous rocell, cur dowly. We can do the reaction record by the addition of a cataly called synes is all The prese change between the land inalconditioned to caluate the world by one mole of standard operate 22.15 Kand preven. For an ideal gas this volumes 22.414 LatSTF Dato's law of Parcial Pressures vates that the rest of mature sels the sun the partial pressure of the componenter Although it may seem that either hydrogen er over Gware & quipment Bree 100011 Two hole white rubber stopper Bakr 600m Vernier gepresenter Hack rubber stopper Versier Latest Interface termeer as 123 Vorrer temperature robe Graduated cylinder 50 m Weighinguel Syringe om, 10 ml. valve and thing Chemicals & Regent LOM drecho CC 10% w/w Hideal Maresme Melal Active dry yet CAUTIONE ICE Bicol with an Procedure Part-Praditionelle 1 Power on the table power button), The Last op dhe automately Connect tenperature robe to the port and pressure were to CH 2. The Gert op will to the connected to delivereadre will opeor in the Meter ren. The wide Tindad Mod with a whole mwend warf the pressure with is change it tot by clicking on the premureve reading and from the rapomeru select Change united then 2 Determine the valla volume ina 125 termeyerland which the cases will 2 File satire volume of the flade with water and insert the black stopper to displace some of the water Messure out the volume of water remaining in the laskine 50m raduted cylinder Engey the contents of the fiestand completely dy there with per towel Uite the fun to what 0.01-0.02 Mg and record the mass Muce the Mg in theory 125 Bremerfis Prepare a room temperature water both in at besker. The bath should be deep enough to completely over the pas level in the termeverlast but not the be opper shown 41 Twith while stopper way the neck of tree as close the value on se white stopper by turning the handle siti perpendicular to the valvester 2 Stoly remove the topper for the flad to see pescure in the lack Do not open the two-way valve to release the pressure 10 Examine the pressure data to determine the change in pressure Select Analy/Statisti followed by both Temperature and Pressure Record Primam in pressure and the mean tamperture during the reaction 11. Discard the contents of the face in the weste container provided in and dry the wide of the flask with paper towel 12. Perform two more trial. When you we ready to start a new on the Collect and select Dhard to delete the previous Partielle 13. Rocca al coop of you in the dy 125 mlrlenmeyer fuck 14. Olain about 12 mt 20 H 3.01 ) Insomt beter. Drew 30 of the HO Into the ringe Sexure the shop the neck of the fuck and the wing Into the TE 15 Repeat the necessary step outlined in Port to determine the pressure change de total CAUTION Moh premre ha generated secure the stepper tightly during the reaction and 16. The content of the flask wat and maybe wached down the link 17. Perform total of three trial * Urolig bh the temperature and the pressure robes bo wings with water and tutun shum to the front. Figura 41 Covprent set with the Versier preure root and temperature probe Obtain 20 LO MC 50 ob Draw 3.0 to the 10 and we carly thread the wingerds the way value on the white show Place them and temperature probe in the water both This step remareste people one to handle the winged the other of hold down the rubber per te that does not pop out of the face when the return capas, the partma to reportis, Top Collect to start the data collection Altersbout 20 seconds, open the mouw valve directly below the wing and slowly pre the plunger to add to the most Quickly close the two-way valve Garty wet the fasthern proceed while down the oper Presepto end data collection once the pressure begin to drop At this point all of de Method 9. 10. Slowly remove the white stopper from the flask to relieve pressure in the flask. Do not open the two-way valve to release the pressure Examine the pressure data to determine the change in pressure Select Analyze/Statisties followed by both Temperature and Pressure. Record Prot (max. minus min. pressure) and the mean temperature during the reaction. Discard the contents of the flask in the waste container provided. Rinse and dry the inside of the flask with paper towel. 12. Perform two more trials. When you are ready to start a new run, tap Collect and select Discard to delete the previous run. Part 8 - Production of Orle 13. Place a small scoop of yeast in the dry 125 mL Erlenmeyer flask. 14. Obtain about 12 mt 3.0 % w/w H:02 (d = 1.01 g/ml) in a 50 ml beaker. Draw 3.0 ml of the H20; into the 3 ml syringe. Secure the stopper in the neck of the flask and the syringe into the valve. 15. Repeat the necessary steps as outlined in Part A to determine the pressure change due to Oale CAUTION: High pressure is generated; secure the stopper tightly during this reaction and be very careful when releasing the pressure The content of the flask (yeast and Hu0) may be washed down the sink. Perform a total of three trials. 18. Unplug both the temperature and the pressure probes. 19. Rinse both syringes with water and return them to the front. 16. 17. Volume of water occupying the flask (v.):__137m1_ Volume of produced gas (V+) =Vw + volume of gas occupying tubing and sensor volume of added acid or H2O2: (0.25 Marks) Water bath mean temperature: _24.0C Vapour pressure (atm) of water at water bath mean temp. from CRC hand book_22.377 He_kPa _atm (0.25 Marks) tttt Table 4.1 Trial Mass of Mg 0.017 0.017 0.0169 Mol of Hamile P113.01kPa 113.11kPa 112.91kPa Pon99.27kPa 99.31kPa 99.13kPa PP-P) P.PH 3 Mean temp., K (T) 24.02 C 24.02 C 24.02 C Volume Hz at STP. (Va) Average: Calculations: Complete Table 4.1, using the correct units calculate the average molar volume of Heat STP. V. Where ns is the moles of gas produced based on the amount of limiting reactant and the reaction stoichiometry, and P, is the pressure of dry gas (see Eq. 3). Show all of your work. (3.0 Marks) Part 8 - Molar Volume of O, Give the balanced reaction equation: (0.25 Marks Table 4.2 Trial Vol. HO 3ml 3ml 3m Mol Ol P112,04P 120.01 124.91P P97.27 Pa 96.31P 98.13 Pa PPP Po.PH Mean temp. (T) 24.4 24.6" - 24.32 Volume 03 at STP. (2) Average Note: If you notice an outlier in the results, you can exclude that from calculating the werage volume of the gas at STP. Question: Discuss why the value of one of the trials cannot be used in the calculation of the average volume of Oz gas and give the possible reason for getting this out of the range value. (0.75 marks Calculations: Complete Table 4.2 using the correct units calculate the average molar volume of Oz at STP, V. Where na is the moles of gas produced based on the volume concentration and density of H:Os as well as the reaction stoichiometry. Show all of your work 3.0 Marks Calculate the error of the molar volume of Oz. (0.25 Marks) - Calculate the density (in g/L) of both hydrogen and oxygen gas from average molar volumes at STP. (1.0 Marks) Hypothesis [2.0] A hypothesis summarizes what outcomes you anticipate for the experimental procedure. Typically the outcomes will be presented in terms of the relationship between dependent and independent variables. Give the reason for your hypothesis based on what you know about the scientific concept of the lab and how that knowledge led you to the hypothesis. Discussion (3.0) Explain how the data supports, or does not support, your hypothesis. Discuss the accuracy (% error) between experimental and expected values in regards to molar volume and density. Identify at least one source of error. How does this error affect the data and how could it be improved
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


