Question: The Scenario You and your colleagues in Professor Ndekele's lab get samples of a newly discovered element to characterize! A co-researcher in another lab has
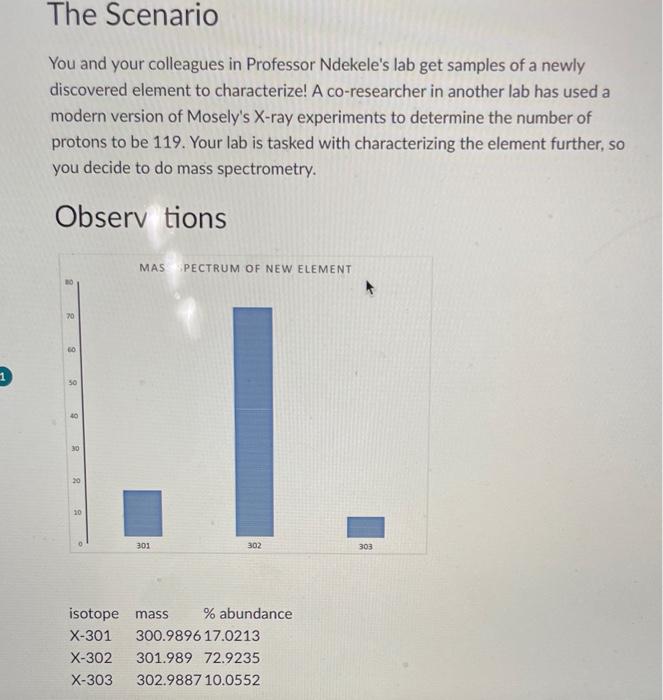

The Scenario You and your colleagues in Professor Ndekele's lab get samples of a newly discovered element to characterize! A co-researcher in another lab has used a modern version of Mosely's X-ray experiments to determine the number of protons to be 119. Your lab is tasked with characterizing the element further, so you decide to do mass spectrometry. Observ tions Analysis \& Suiomission "Submit one .pdf or doc file that contains the answers to all of the quistions below in complete sentences. Show all calculations, including units 1. How many isotopes are there and how do you know from the mass spectrum? 2. Calculate the average atomic mass of the element, showing your work. 3. Determine how many protons, neutrons and electrons are present in each isotope. 4. Professor Ndekele gives you the honor of naming this new element. Name it, then construct a periodic table entry that includes the atomic number, the element symbol (two unique letters corresponding to the name, but different from any others. The Scenario You and your colleagues in Professor Ndekele's lab get samples of a newly discovered element to characterize! A co-researcher in another lab has used a modern version of Mosely's X-ray experiments to determine the number of protons to be 119. Your lab is tasked with characterizing the element further, so you decide to do mass spectrometry. Observ tions Analysis \& Suiomission "Submit one .pdf or doc file that contains the answers to all of the quistions below in complete sentences. Show all calculations, including units 1. How many isotopes are there and how do you know from the mass spectrum? 2. Calculate the average atomic mass of the element, showing your work. 3. Determine how many protons, neutrons and electrons are present in each isotope. 4. Professor Ndekele gives you the honor of naming this new element. Name it, then construct a periodic table entry that includes the atomic number, the element symbol (two unique letters corresponding to the name, but different from any others
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


