Question: This problem continues Exercises 1 and 2. However, note that the initial volume and temperature may have changed due to variable randomization. Pay attention to
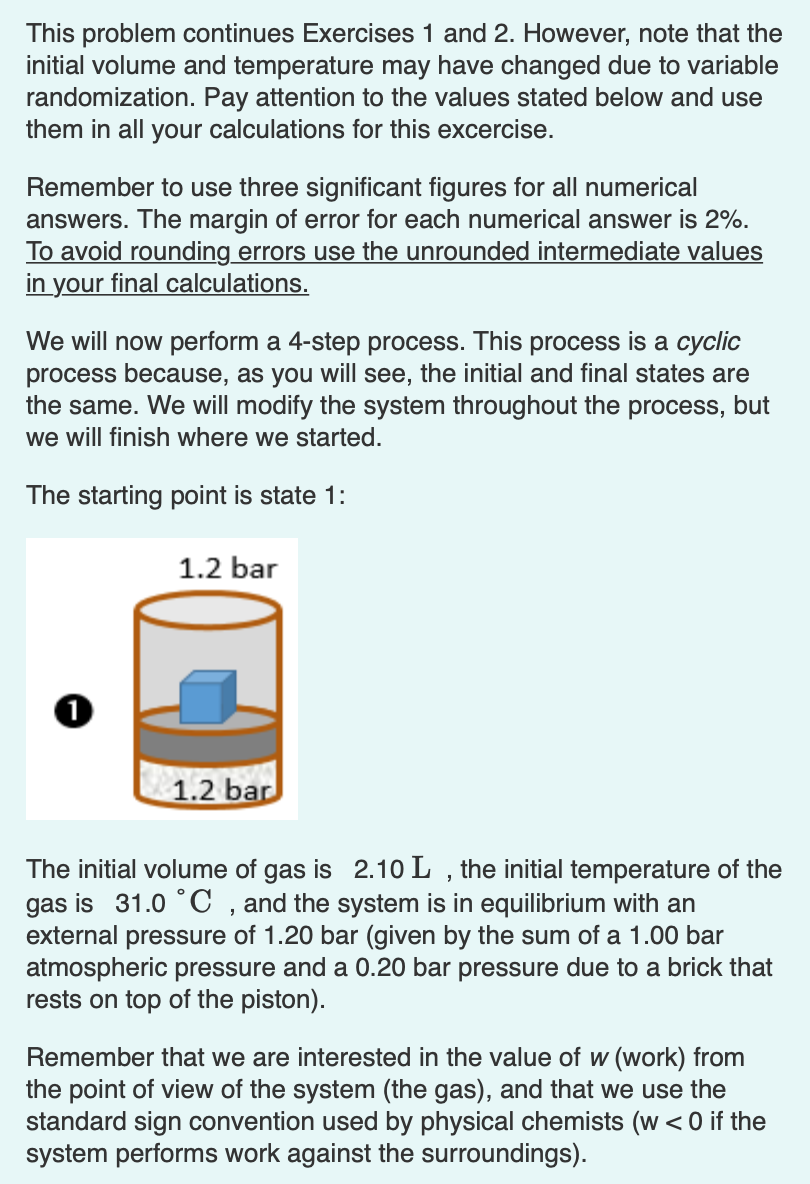
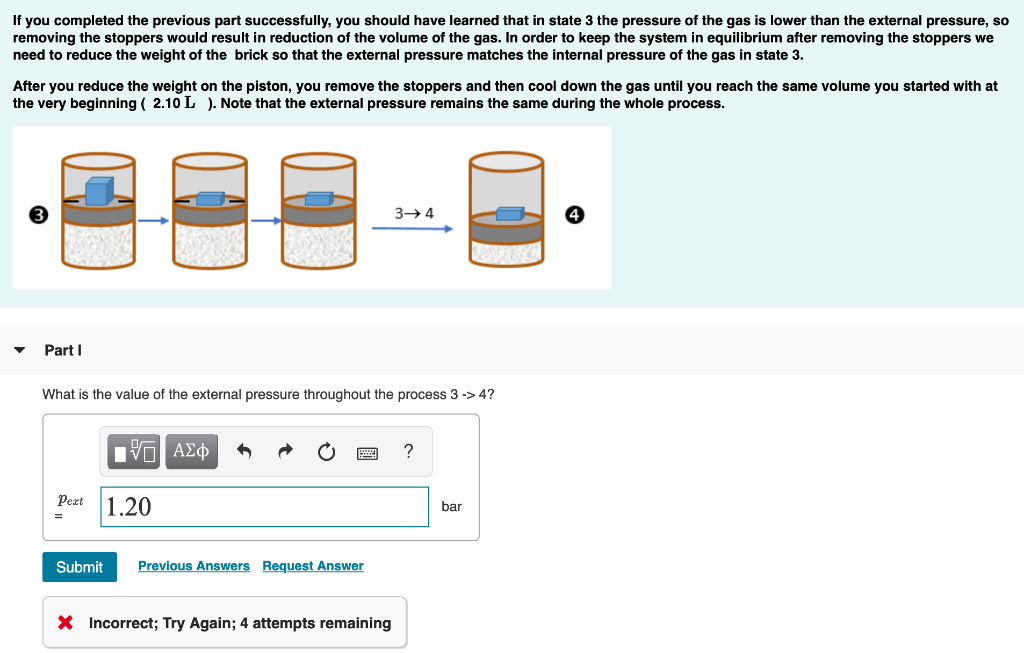
This problem continues Exercises 1 and 2. However, note that the initial volume and temperature may have changed due to variable randomization. Pay attention to the values stated below and use them in all your calculations for this excercise. Remember to use three significant figures for all numerical answers. The margin of error for each numerical answer is 2%. To avoid rounding errors use the unrounded intermediate values in your final calculations. We will now perform a 4-step process. This process is a cyclic process because, as you will see, the initial and final states are the same. We will modify the system throughout the process, but we will finish where we started. The starting point is state 1: 1.2 bar 1 1.2 bar The initial volume of gas is 2.10 L , the initial temperature of the gas is 31.0C , and the system is in equilibrium with an external pressure of 1.20 bar (given by the sum of a 1.00 bar atmospheric pressure and a 0.20 bar pressure due to a brick that rests on top of the piston). Remember that we are interested in the value of w (work) from the point of view of the system (the gas), and that we use the standard sign convention used by physical chemists (w 4? VO AE ? Pest 1.20 bar Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part J What is the temperature of the gas after you complete step 3 -> 4? ? T = C Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part K What is the value of w for process 3-4? Think about the sign first! IVO AE W= J Submit Request Answer This problem continues Exercises 1 and 2. However, note that the initial volume and temperature may have changed due to variable randomization. Pay attention to the values stated below and use them in all your calculations for this excercise. Remember to use three significant figures for all numerical answers. The margin of error for each numerical answer is 2%. To avoid rounding errors use the unrounded intermediate values in your final calculations. We will now perform a 4-step process. This process is a cyclic process because, as you will see, the initial and final states are the same. We will modify the system throughout the process, but we will finish where we started. The starting point is state 1: 1.2 bar 1 1.2 bar The initial volume of gas is 2.10 L , the initial temperature of the gas is 31.0C , and the system is in equilibrium with an external pressure of 1.20 bar (given by the sum of a 1.00 bar atmospheric pressure and a 0.20 bar pressure due to a brick that rests on top of the piston). Remember that we are interested in the value of w (work) from the point of view of the system (the gas), and that we use the standard sign convention used by physical chemists (w 4? VO AE ? Pest 1.20 bar Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part J What is the temperature of the gas after you complete step 3 -> 4? ? T = C Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining Part K What is the value of w for process 3-4? Think about the sign first! IVO AE W= J Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


