Question: U = 3/2nRT monatomic u = 5/2 nRT diatomic Processes PV diggram work done heat added/ removed P I so baric W = PAVE AV=
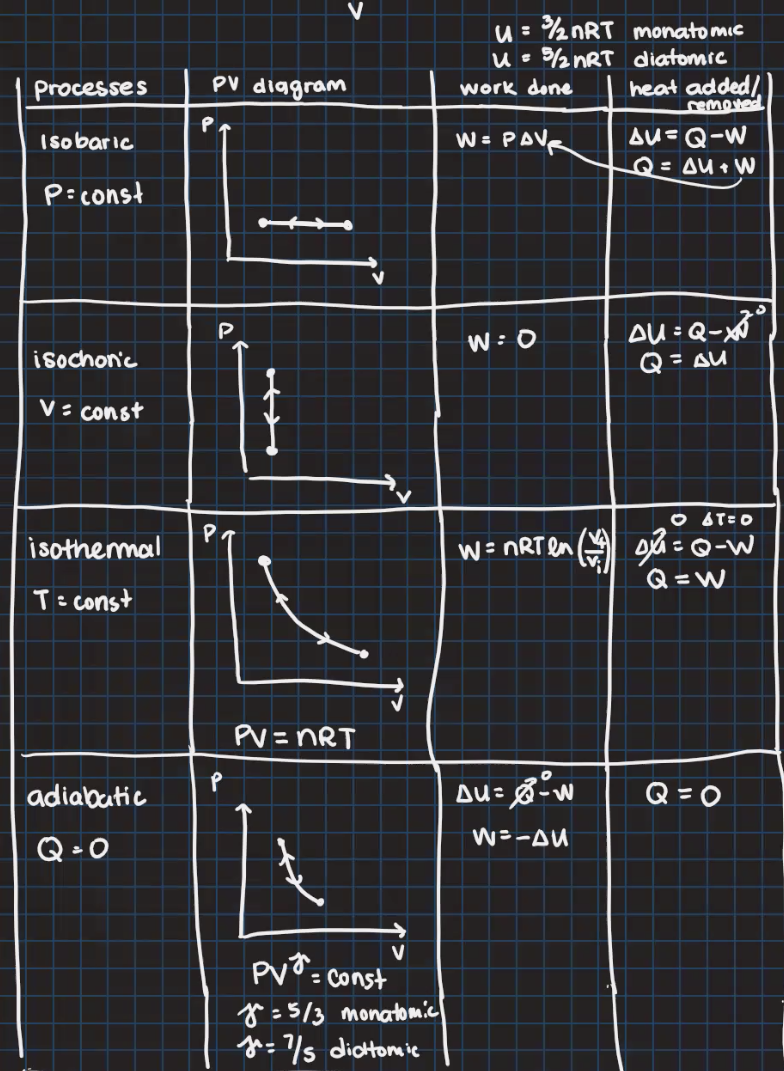
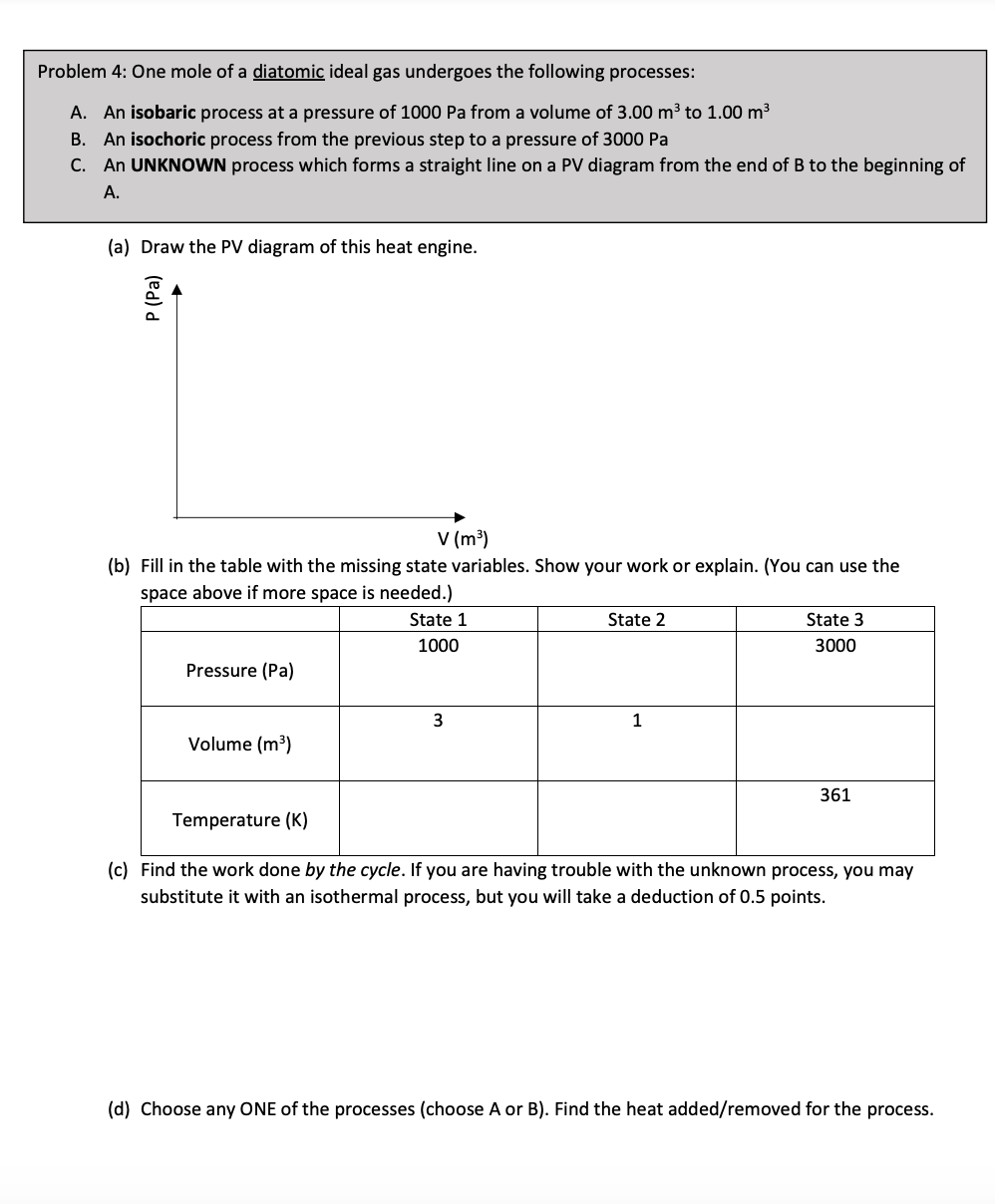
U = 3/2nRT monatomic u = 5/2 nRT diatomic Processes PV diggram work done heat added/ removed P I so baric W = PAVE AV= Q-W Q= AU + W P : const P W : D DU = Q-X isochon'c Q = AU V = const PT O &TED isothermal W= nRTen () DM = Q -W Q = W T = const PV = nRT adiabatic P Au = 8-w Q = O Q=O W=-AU PV = const 8 : 5 /3 monatomic 1 = 7 /s dicttomicProblem 4: One mole ofa diatomic ideal gas undergoes the following processes: A. An isobaric process at a pressure of 1000 Pa from a volume of 3.00 m3 to 1.00 m3 B. An isochoric process from the previous step to a pressure of 3000 Pa C. An UNKNOWN process which forms a straight line on a PV diagram from the end of B to the beginning of A. (a) Draw the P'v' diagram of this heat engine. au ID a. q...- n. V (m3) (bl Fill in the table with the missing state variables. Show your work or explain. (You can use the space above if more space is needed.) State 1 State 2 State 3 1000 3000 Pressure (Pa) 3 1 Volume [m3] 361 Temperature (K) (c] Find the work done by the cycle. If you are having trouble with the unknown process, you may substitute it with an isothermal process, but you will take a deduction of 0.5 points. (all Choose any ONE of the processes {choose A or B). Find the heat added/removed for the process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


