Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Vaporization of mixtures of hexane and octane. Using the T-x-y diagram (Figure 1) on the next page, determine the temperature, amounts, and compositions of the
Vaporization of mixtures of hexane and octane. Using the T-x-y diagram (Figure 1) on the next page, determine the temperature, amounts, and compositions of the vapor and liquid phases at 1 atm for the following conditions with a 100 kmol mixture of hexane and octane. Use A for hexane and B for octane. Show your calculation steps and include relevant drawings (e.g., tie lines) on the diagram. (a) = 0.5, Ψ = ⁄ = 0.2 (b) = 0.4, = 0.6 (c) = 0.6, = 0.7 (d) = 0.5, Ψ = 0 (e) = 0.5, Ψ = 1 (f) = 0.5, = 200 °F
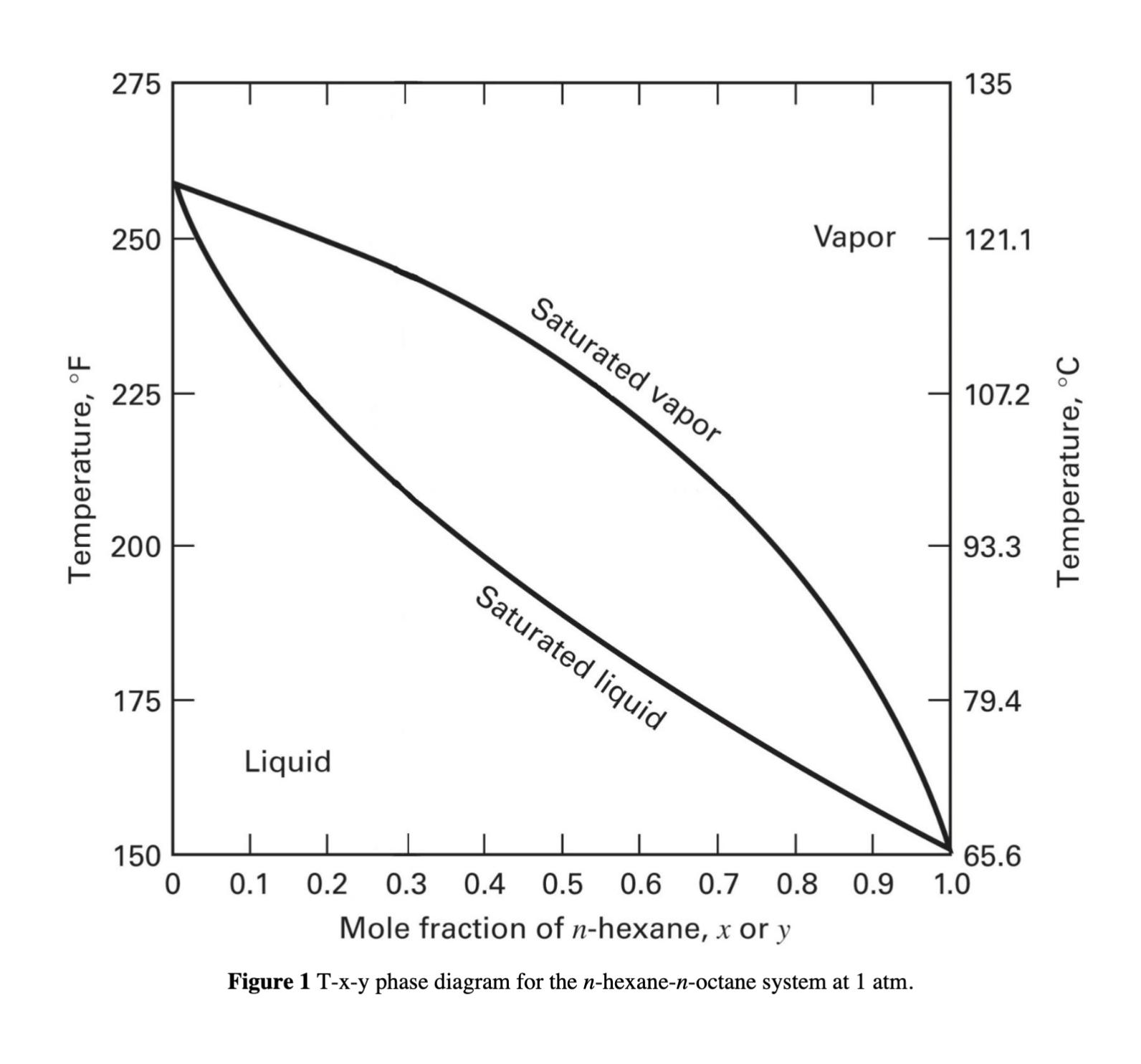
Temperature, F 275 250 225 200 175 Liquid Saturated vapor Saturated liquid 135 Vapor 121.1 107.2 93.3 79.4 150 65.6 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 Mole fraction of n-hexane, x or y 0.9 1.0 Figure 1 T-x-y phase diagram for the n-hexane-n-octane system at 1 atm. C Temperature,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


