Question: We were taking notes on this worksheet in class and I missed 10,11 and 13 (organic chem). I would like help on one or all
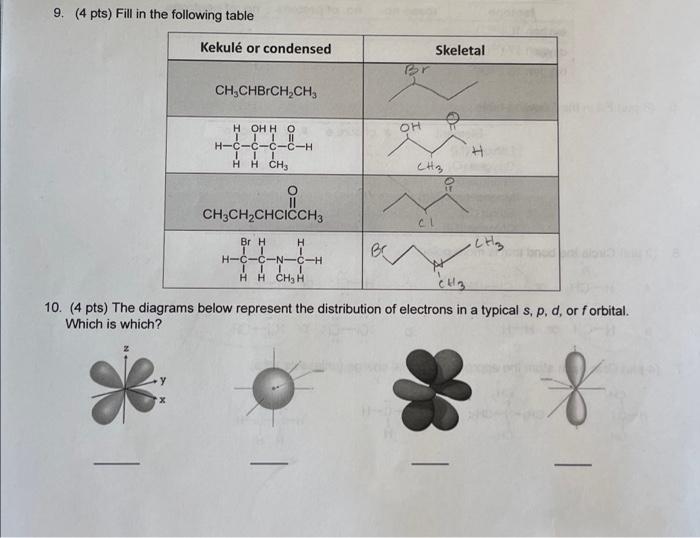

9. (4 pts) Fill in the following table Kekul or condensed Skeletal Br CH,CHBrCH.CH e OH H-6-6-6-6-H !! 1 H H CH H CH3 2 CH3CH2CHCICCHE C1 er .(Hz Br H H HTC CNCH , A 10. (4 pts) The diagrams below represent the distribution of electrons in a typical s, p, d, or forbital. Which is which? X - Chem 270 Worksheet 1 W2002 11. (2 pts) The sigma orbitals in ammonia are formed by overlap of which orbitals? The unshared electron pair on the nitrogen atom inhabits what type of orbital? 12. (4 pts) Hydrogen peroxide (H2O2) is an oxidant a How are the orbitals of the two oxygen atoms hybridized? So ? b. Describe the O-O and O-H bonds of hydrogen peroxide. What orbitals overlap to form them? Are they sigma or pi bonds? Bond orbitals SOP 0-0 O-H 13. c. What type of orbital do the lone-pair electrons inhabit? 0-spe orbital (5 pts) Diazine has the formula NzHz How are the orbitals of the two nitrogen atoms hybridized? Describe the N-N and N-H bonds of diazine. What orbitals overlap to form them? a b. Bond orbitals SOP N-N 5 N-N N-H c. What type of orbitals do the lone-pair electrons inhabit? 14. (2 pts) What are the hybridizations of the C and O atoms in CO ? o=c=0 Hybridization Atom 0 sp sp? and pi bonds lo Csp-Osp la cp - Op // bonding in aethene Sigma stronger than pibond closer to nuclei
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


