Question: WEEK 3 MODULE 3: The Structure of Atom Matter is composed of tiny particles called atom. which consist of charges that can either be positive
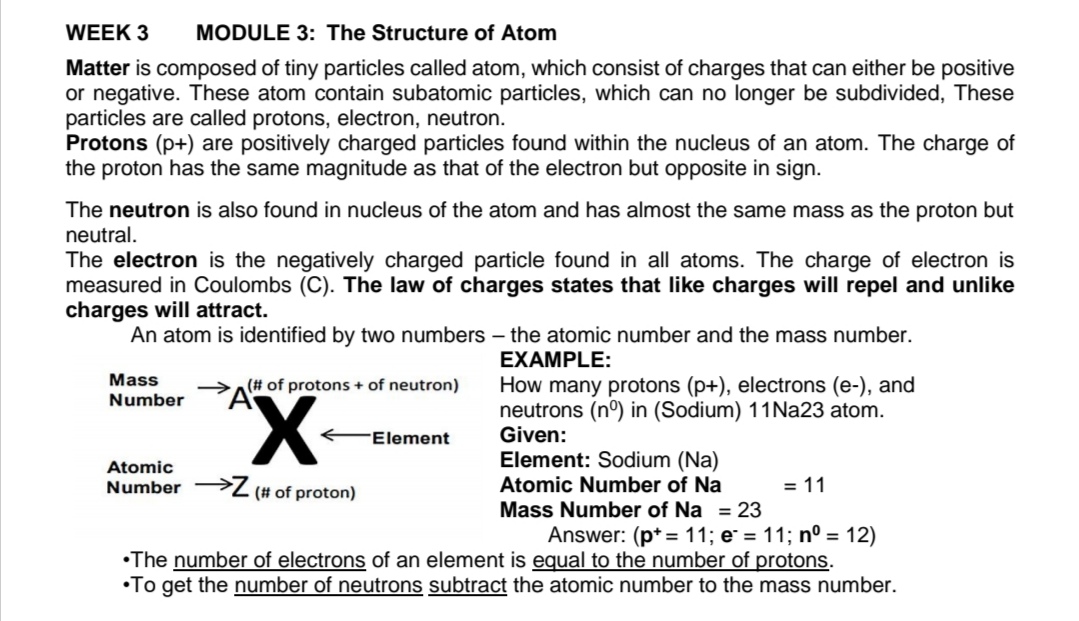
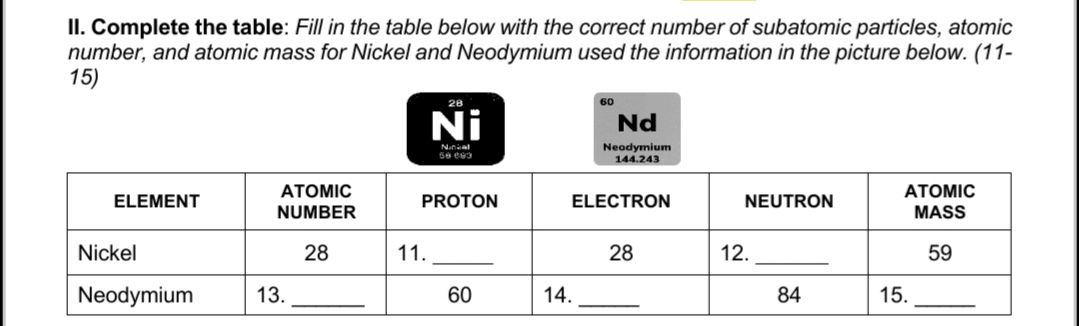
WEEK 3 MODULE 3: The Structure of Atom Matter is composed of tiny particles called atom. which consist of charges that can either be positive or negative. These atom contain subatomic particles. which can no longer be subdivided. These particles are called protons. electron, neutron. Protons (p+) are positively charged particles found within the nucleus of an atom. The charge of the proton has the same magnitude as that of the electron but opposite in sign. The neutron is also found in nucleus of the atom and has almost the same mass as the proton but neutral. The electron is the negatively charged particle found in all atoms. The charge of electron is measured in Coulombs (C). The law of charges states that like charges will repel and unlike charges will attract. An atom is identified by two numbers the atomic number and the mass number. EXAHPLE: \"a\" In 0! protons + of neutron} How many protons (p+). electrons (e-). and "ummra'. x neutrons (no) in (Sodium) 1 1Na23 atom. Z (common) Atomic Number of Na = 11 Mass Number of Na = 23 Answer: (p+=11;e' =11; n'I =12) *The number of electrons of an element is Quai to the number of protons. -To get the number of neutrons subtract the atomic number to the mass number. Il. Complete the table: Fill in the table below with the correct number of subatomic particles, atomic number, and atomic mass for Nickel and Neodymium used the information in the picture below. (11- 15) 28 60 Ni Nd Nankal Neodymium 144.243 ELEMENT ATOMIC PROTON ELECTRON NEUTRON ATOMIC NUMBER MASS Nickel 28 11. 28 12. 59 Neodymium 13. 60 14. 84 15
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


