A cylinder of ideal gas is closed by an 8.00 kg movable piston (area = 60.0 cm
Question:
A cylinder of ideal gas is closed by an 8.00 kg movable piston (area = 60.0 cm2) as illustrated in Fig. 20-5. Atmospheric pressure is 100 kPa. When the gas is heated from 30.0 °C to 100.0 °C, the piston rises 20.0 cm. The piston is then fastened in place, and the gas is cooled back to 30.0 °C. Calling ΔQ1 the heat added to the gas in the heating process, and ΔQ2 the heat lost during cooling, find the difference between ΔQ1 and ΔQ2.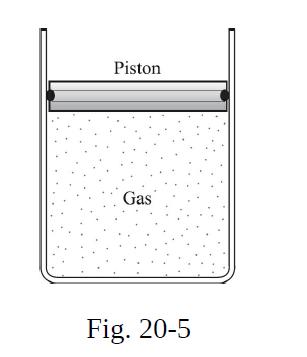
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





