Ethanol and benzene are separated in a network of distillation and membrane separation steps. In one step,
Question:
Ethanol and benzene are separated in a network of distillation and membrane separation steps. In one step, a near-azeotropic liquid mixture of 8,000 kg/h of 23 wt% ethanol in benzene is fed to a pervaporation membrane consisting of an ionomeric film of Ethanol and benzene are separated in a network of distillation and membrane separation steps. In one step, a near-azeotropic liquid mixture of 8,000 kg/h of 23 wt% ethanol in benzene is fed to a pervaporation membrane consisting of an ionomeric film of perfluorosulfonic polymer cast on a Teflon support. The membrane is selective for ethanol, so the vapor permeate contains 60 wt% ethanol, while the non-permeate liquid contains 90 wt% benzene.
(a) Draw a flow diagram of the pervaporation step using symbols from Table 1.2, and include all process information.
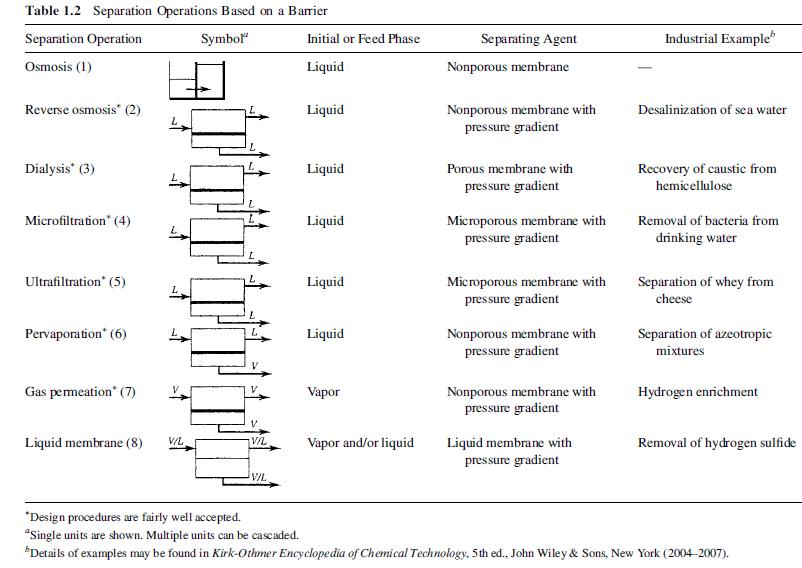 (b) Compute the component flow rates in kg/h in the feed stream and in the product streams, and enter these results into the diagram.
(b) Compute the component flow rates in kg/h in the feed stream and in the product streams, and enter these results into the diagram.
(c) What operation could be used to purify the vapor permeate?
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





