Fifty mol% methanol in water at 101 kPa is continuously distilled in a seven-plate, perforated-tray column, with
Question:
Fifty mol% methanol in water at 101 kPa is continuously distilled in a seven-plate, perforated-tray column, with a total condenser and a partial reboiler heated by steam. Normally, 100 kmol/h of feed is introduced on the third plate from the bottom. The overhead product contains 90 mol% methanol, and the bottoms 5 mol%. One mole of reflux is returned for each mole of overhead product. Recently it has been impossible to maintain the product purity in spite of an increase in the reflux ratio. The following test data were obtained:
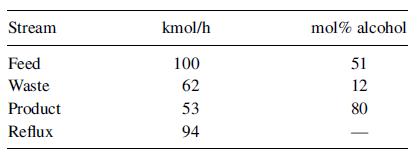
What is the most probable cause of this poor performance? What further tests would you make to establish the reason for the trouble? Could some 90% product be obtained by further increasing the reflux ratio, while keeping the vapor rate constant?
Vapor–liquid equilibrium data at 1 atm [Chem. Eng. Prog., 48, 192 (1952)] in mole-fraction methanol are
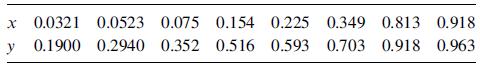
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





