Consider these six-coordinate ionic radii for divalent, first-row transition-metal ions: r(Ti+) = 0.86 , r(V+) = 0.79
Question:
Consider these six-coordinate ionic radii for divalent, first-row transition-metal ions:
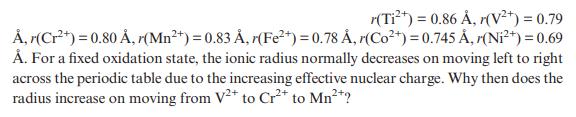
Transcribed Image Text:
r(Ti+) = 0.86 , r(V+) = 0.79 , r(Cr+) = 0.80 , r(Mn+) = 0.83 , r(Fe+) = 0.78 , r(Co+) = 0.745 , (Ni+) = 0.69 . For a fixed oxidation state, the ionic radius normally decreases on moving left to right across the periodic table due to the increasing effective nuclear charge. Why then does the radius increase on moving from V2+ to Cr+ to Mn+?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The deviation from the general trend is due to occupation ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
Write a policy statement as the HR director stating whether or not office romantic relationships are allowed. If so, under what circumstances? What theoretical ethical perspective did you use to...
-
(a) Using data from Appendix 6, plot a graph to show how the ionic radii of high-spin, 6-coordinate M 2+ ions of the first row of the d-block vary with the d n configuration. Comment on factors that...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Your company, Printers Inc., is considering investing in a new plant to manufacture a new generation of 3D printers developed by the firms research and development (R&D) department. A consulting...
-
Determine by direct integration the product of inertia of the given area with respect to the x and y axes. y= ketla
-
The article referred to in Exercise 23 presents values for the dependent and independent variables for 10 additional construction jobs. These values are presented in Tables SE24A and SE24B (page...
-
1. To develop an understanding of your ethical leadership style 2. To understand how your preferred ethical leadership style relates to other ethical leadership styles Directions 1. Please read the...
-
Warranties Winslow Company sold 150 color laser copiers in 2010 for $4,000 apiece, together with a one-year warranty. Maintenance on each copier during the warranty period averages $300. (a) Prepare...
-
thank you I've been having lots of troubles opening any documents in full they just appear as blank would you know why this is or perhaps be able to assist me
-
For which d-electron counts are there distinct HS and LS states of an octahedrally coordinated transition-metal ion?
-
Construct an MO diagram for trigonal-planar BH3 by analogy with the MOs for trigonal-planar NH 3 in Figure 5.26. Use this diagram to determine the degeneracy and orbital character of the HOMO and the...
-
Why do individuals and organisations tend to resist change? To what extent do you believe such resistance can be effectively overcome?
-
What are the Social media tools used by Disney ? What is the examination of how those tools support marketing and PR objectives? What is the examination of how the organization effectively uses those...
-
Consider an investor who contacts his/her broker on June 5th to enter into short position on 3 December soybean futures contract. Each contract size is 50lbs. Initial margin requirement is $5000 per...
-
The role-play assignment required you to make "cold calls". There's always a balance between preparing and just picking up the phone and dialing out. How much planning do you do before just picking...
-
1. How does the Whistle-Blower law protect nurses? 2. What are the main goals of a risk management program? 3. describe the role and importance of an incident report? 4. An 87-year-old patient with...
-
The following questions will give you a chance to self-evaluate, to think about what you've been learning in this course, and to draw your own conclusions about how you can apply the skills of...
-
Resnor, Inc., has an issue of preferred stock outstanding that pays a $5.50 dividend every year in perpetuity. If this issue currently sells for $108 per share, the required return is what percent?
-
Starr Co. had sales revenue of $540,000 in 2014. Other items recorded during the year were: Cost of goods sold ..................................................... $330,000 Salaries and wages...
-
Predict which of the following compounds is more acidic, and explain your choice. N- -N- -NH2 NH2
-
Consider the reaction below. The rate of this reaction is markedly increased if a small amount of sodium iodide is added to the reaction mixture. The sodium iodide is not consumed by the reaction and...
-
Below are the structures of two common over-the-counter pain relievers. Determine the hybridization state of each carbon atom in these compounds: a. b. TH. H H H. .C. TH. .C: .. `H Acetylsalicyclic...
-
Solve detailly on excel with formulas UIBS SR United International Business Schools EUROPEAN college for liberal studies WORKING CAPITAL MANAGEMENT GROUP ASSIGNMENT For this Group Assignment, worth...
-
6 8. Write a regular expression whose language is equivalent to the following NFA. start a,b 2 a,b a,b
-
What will be displayed in the list box? for (int i = 2; i < 5; i++) { } outputListBox.Items.Add(i*i);

Study smarter with the SolutionInn App


