The conductivities and crystal structures of the heavier s-block metals are given in the table below. (a)
Question:
The conductivities and crystal structures of the heavier s-block metals are given in the table below.
(a) Calculate the valence-electron concentration n in m−3 and arrange the elements from lowest to highest n.
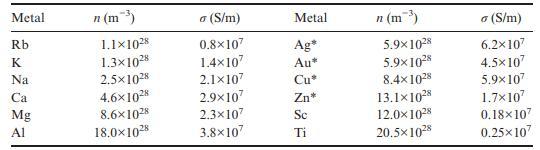
(b) Use these values and those for lighter s-block elements Na, K, Mg, and Ca in Table 10.2 to construct a plot of electrical conductivity (in S/m) versus valence-electron concentration (in m−3).
(c) What can you say about how well these metals follow the predictions of the Drude model?
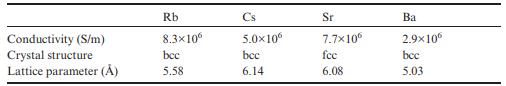
Table 10.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





