Use the ClausiusMossotti expression in Equation (8.13) and Table 8.2 to estimate permittivities of SnO 2 (rutile-type,
Question:
Use the Clausius–Mossotti expression in Equation (8.13) and Table 8.2 to estimate permittivities of SnO2 (rutile-type, P42/mnm, Z = 2, a = 4.74 Å, c = 3.19 Å), TiO2 (rutile-type, a = 4.59 Å, c = 2.96 Å) and ZrO2 (baddeleyite-type, P21/c, Z = 4, unit-cell volume = 141 Å). Does the Clausius–Mossotti equation give a reasonably accurate estimate for each compound when compared to the experimental values of εr given in Table 8.1? If not, what is the origin of the discrepancy?
Equation (8.13)
![a= V 4 2 Er (+2) [CGSes : a in , V, in A]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/9/2/1/12165ae4a61b74551705921119175.jpg)
Table 8.2
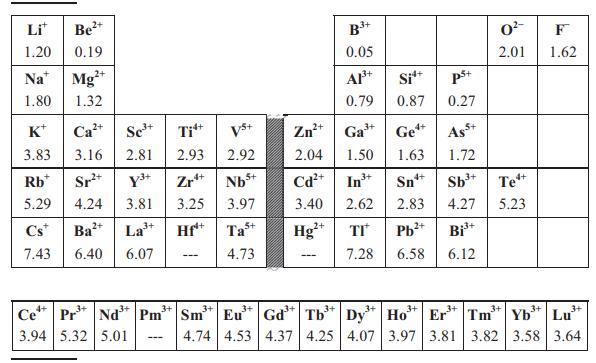
Table 8.1
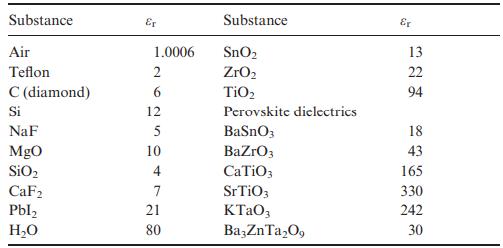
Transcribed Image Text:
a= V 4 2 Er (+2) [CGSes : a in , V, in A]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Calculated values are r 13 SnO 2 r 20 ZrO 2 and r 43 TiO 2 Th...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:
Students also viewed these Sciences questions
-
A researcher wanted to find out if there was difference between older movie goers and younger movie goers with respect to their estimates of a successful actors income. The researcher first...
-
Can you help assist me with an outline for the following essay on supervisory role with the following topics being addressed. I am just looking for an outline as my starting point. THANKS If given...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
ABC Insurance Company has issued a commercial package policy to the Henderson Company. ABC recently discovered that company executives misrepresented important information about the business to...
-
As shown in Fig.P2.39, a steel wire suspended vertically having a cross-section area A and an initial length X0 is stretched by a downward force F applied to the end of the wire. The normal stress in...
-
Many cancers seem to involve environmental factors. Why, then, is cancer called a genetic disease?
-
Consider the following cash flow profile and assume MARR is 10 percent/year and the finance rate is 4 percent/year. a. Determine the MIRR for this project. b. Is this project economically attractive?...
-
Bill's Barbershop has two barbers available to cut customers' hair. Both barbers provide roughly the same experience and skill, but one is just a little bit slower than the other. The process flow in...
-
Mr. Amar is 30 year old, newly married and a successful actor in the Indian film industry. Right from his struggling days, Amar always saved a part of his income and invested in safe instruments like...
-
Why doesthe polarizability of the lanthanoid ions decrease as the atomic numberincreases?
-
Derive the ClausiusMossotti equation for the CGSes system of units.
-
Comet operates solely within the United States. It owns two subsidiaries conducting business in the United States and several foreign countries. Both subsidiaries are U.S. corporations. This year,...
-
In the summer of 2017, Reigh Webster and her friend Ella Hill were relaxing in a local watering hole complaining about their boring monotonous jobs. Reigh told Ella that she has been saving her money...
-
Your broker charges $0.0025 per share per trade. The exchange charges $0.0149 per share per trade for removing liquidity and credits $0.0131 per share per trade for adding liquidity. The current best...
-
A pendulum has a period of 1.59 s on Earth. What is its period on Mars, where the acceleration of gravity is about 0.37 that on Earth? Express your answer to two significant figures and include the...
-
4. Calculate the NOPAT, given the following information: Net profit: 4000 Interest: 1000 Tax: 1000 Loss from foreign currency devaluation: 2000 Gain from non-operational investments: 2000 Enter...
-
Besides the destructive trade in enslaved people, why, in Afonso's words, has the economic relationship with Portuguese merchants been harmful to Afonso's kingdom?
-
Presented below is the balance sheet of Bellemy Brothers Corporation (000s omitted). Instructions Evaluate the balance sheet presented. State briefly the proper treatment of any itemcriticized....
-
Modify the CYK algorithm so that it applies to any CFG, not just those in CNF.
-
Discuss the following statement: If the temperature of the system increased, heat must have been added to it.
-
Oxygen reacts with solid glycylglycine C 4 H 8 N 2 O 3 to form urea CH 4 N 2 O, carbon dioxide, and water: 3O 2 (g) + C 4 H 8 N 2 O 3 (s) CH 4 N 2 O(s) + 3CO 2 (g) + 2H 2 O(l) At T = 298 K and 1.00...
-
Identify the reagents that you would use to achieve each of the following transformations: a. b. Br Br
-
Jupiter has 67 confirmed moons. Four of the sixty-seven dominate the others (in terms of mass) and data of these four are given in the table. Based on the information given, calculate the mass of...
-
In the upper 10 km of the crust, the geothermal gradient is typically about 25C per km, but it can range from 15C/km to 50C/km. In the chart below, plot these three geothermal gradients (15C/km,...
-
4. If the feature vector is three-dimensional, the perceptron has four parameters. Suppose all parameters are in the range [-1,1] and we search this range by dividing it by 0.0001 intervals. (1)...

Study smarter with the SolutionInn App


