(a) The 1 H NMR spectrum of [18]annulene shows two signals, at = 9.28 (12 H)...
Question:
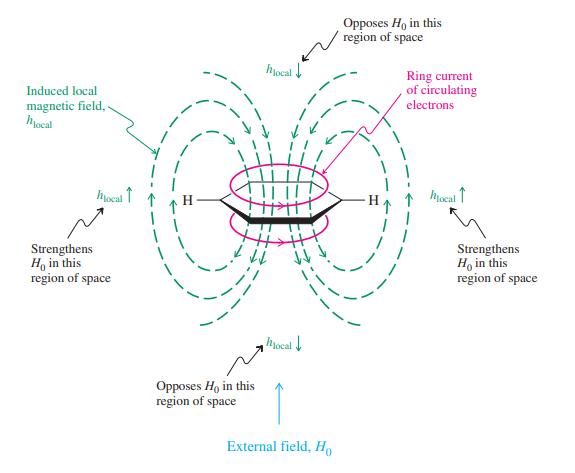
(a) The 1H NMR spectrum of [18]annulene shows two signals, at δ = 9.28 (12 H) and -2.99 (6 H) ppm. The negative chemical shift value refers to a resonance upfield (to the right) of (CH3)4Si. Explain this spectrum. (Consult Figure 15-9.) (b) The unusual molecule 1,6-methano[10]annulene (shown in the margin) exhibits two sets of signals in the 1H NMR spectrum at δ = 7.10 (8 H) and -0.50 (2 H) ppm. Is this result a sign of aromatic character?
![H H 1,6-Methano[10]annulene](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1589/9/5/2/9965ec4c1e4dfc261589952993368.jpg)
Figure 15-9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





