Given the enthalpy of formation for food waste to be (-394.7 mathrm{MJ} / mathrm{kmol}), use the combustion
Question:
Given the enthalpy of formation for food waste to be \(-394.7 \mathrm{MJ} / \mathrm{kmol}\), use the combustion reaction from Equation 4.11 to calculate its HHV (assume that the molecular formula is the same as switchgrass). Repeat this calculation for redwood (assume an enthalpy of formation of \(-50.7 \mathrm{MJ} / \mathrm{kmol})\).
Equation 4.11
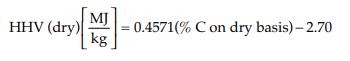
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





