The saturation domes for R-134a and isopentane shown in Figures 13.4 and 13.18? are quite asymmetric, with
Question:
The saturation domes for R-134a and isopentane shown in Figures 13.4
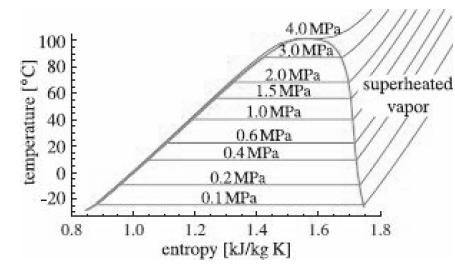
and 13.18?
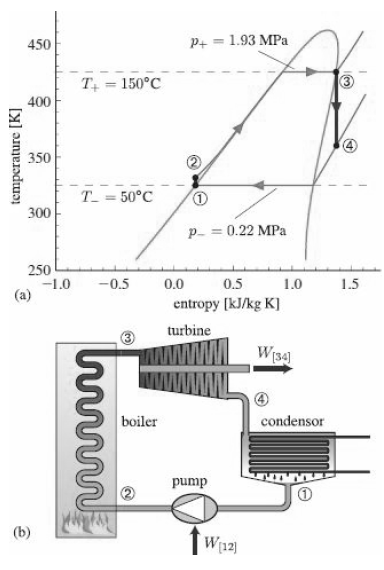
are quite asymmetric, with steep (R-134a) or even re curving (isopentane) saturation curves. Water, on the other hand, has a relatively symmetric saturation dome (Figure 12.9)
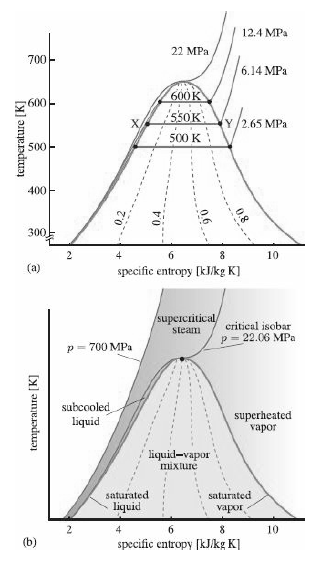
Explain why this feature makes R-134a and isopentane more suitable fluids for low-temperature heat extraction devices and engines than water. Why is water so widely used for higher-temperature systems such as steam turbines? What kind of shape for the saturation dome would be better for a phase-change fluid in a higher-temperature system?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





